
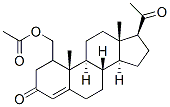
| Name | Medroxyprogesterone Acetate |
|---|---|
CAS | 71-58-9 |
Synonyms | 17a-hydroxy-6a-methyl-4-pregnene-3,20-dione acetate 17alpha-acetoxy-6-alpha-methylpregn-4-ene-3,20-dione 17ALPHA-ACETOXY-6ALPHA-METHYLPROGESTERONE 17ALPHA-HYDROXY-6ALPHA-METHYL-4-PREGNENE-3,20-DIONE 17-ACETATE 17-ALPHA-HYDROXY-6-ALPHA-METHYL-4-PREGNENE-3,20-DIONE 17-ALPHA-ACETATE 4-PREGNEN-6ALPHA-METHYL-17ALPHA-OL-3,20-DIONE 17-ACETATE 4-PREGNEN-6-ALPHA-METHYL-17-OL-3,20-DIONE ACETATE 6-ALPHA-METHYL-17-ACETOPROGESTERONE 6ALPHA-METHYL-17ALPHA-ACETOXYPROGESTERONE 6ALPHA-METHYL-17ALPHA-HYDROXY-PROGESTERONE ACETATE DEPO-PROVERA hydroxymethylprogesterone acetate MEDROXYPROGESTERONE 17-ACETATE MEDROXYPROGESTERONE ACETATE MPA PROVERA (6-alpha)-17-(acetyloxy)-6-methylpreg-4-ene-3,20-dione 17-(acetyloxy)-6-methyl-(6-alpha)-pregn-4-ene-20-dione 17-(acetyloxy)-6-methyl-20-dion(6alpha)-pregn-4-ene- 17-acetoxy-6-alpha-methylprogesterone |
EINECS(EC#) | 200-757-9 |
[Molecular Formula | C24H34O4 |
MDL Number | MFCD00010483 |
Molecular Weight | 386.52 |
| MOL File | 71-58-9.mol |
| Appearance | white crystalline powder |
| mp | 206-207 °C(lit.) |
| refractive index | 48 ° (C=1, Dioxane) |
| Stability | Stable, but weakly air and light sensitive. Incompatible with strong oxidizing agents. |
| Water Solubility | <0.1 g/100 mL at 23 ºC |
| Merck | 13,5817 |
| CAS DataBase Reference | 71-58-9(CAS DataBase Reference) |
| EPA Substance Registry System | 71-58-9(EPA Substance) |

Medroxyprogesterone Acetate Chemical Properties | |
|---|---|
Melting point | 206-207 °C(lit.) |
alpha | D +61° (in chloroform) |
Boiling point | 432.7°C (rough estimate) |
density | 1.0346 (rough estimate) |
refractive index | 48 ° (C=1, Dioxane) |
storage temp. | Refrigerator |
solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in acetone, sparingly soluble in ethanol (96 per cent) |
form | neat |
Water Solubility | <0.1 g/100 mL at 23 ºC |
Merck | 13,5817 |
BRN | 2066112 |
Stability: | Stable, but weakly air and light sensitive. Incompatible with strong oxidizing agents. |
CAS DataBase Reference | 71-58-9(CAS DataBase Reference) |
IARC | 2B (Vol. 21, Sup 7) 1987 |
EPA Substance Registry System | Medroxyprogesterone acetate (71-58-9) |
Safety Information | |
|---|---|
Hazard Codes | Xn |
Risk Statements | 40-48 |
Safety Statements | 22-36/37/39-45 |
WGK Germany | 3 |
RTECS | TU5010000 |
HS Code | 29372390 |
Medroxyprogesterone Acetate Usage And Synthesis | |
|---|---|
Chemical Properties | White or almost white, crystalline powder. |
Originator | Provera,Upjohn,US,1959 |
Uses | Progestogen; an injectable contraceptive. |
Uses | antiprotozoal |
Uses | A synthetic progesterone receptor agonist |
Manufacturing Process | Preparation of 17α-Hydroxyprogesterone 3,20-Bis-(Ethylene Ketal): A solution was prepared containing 50.0 g of 17α-hydroxyprogesterone in 1,000 ml of benzene, 100 ml of ethylene glycol and 2.5 g of p-toluenesulfonic acid monohydrate. This mixture was refluxed for a period of 17 hours using a calcium carbide water-trap to remove the water formed in the reaction. After this period of reflux 6.5 ml of pyridine was added to the solution, and the mixture cooled to room temperature. |
Brand name | Amen (Amarin); Curretab (Solvay Pharmaceuticals); Cycrin (ESI); Provera (Pharmacia & Upjohn);Clinovie;Cliovir;Dep0-clinover;Dep0-map;Depcorlutin;Depo-prodasone;Depo-progevera;Depo-promone;Deporone;Dugen;Farlurin;Farlutale;Gesinal;Gestapuran;Gestapuron;G-farlutal;Hysron;Intex;Luteocrin orale;Luteodione;Luteos;Lutoporal;Metigestene;Nadigest;Nogest;Onco-provera;Perlutest;Petogen;Piermap;Povera;Promone-e;Pronone;Proverone;Provest;Sindomens;Sodelut "g";Supprestal;Verafen;Veramix plus v. |
Therapeutic Function | Progestin |
World Health Organization (WHO) | A depot preparation containing 150 mg medroxyprogesterone acetate was introduced over 20 years ago for use as a long-acting injectable contraceptive. |
General Description | MPA, 17-acetyloxy-6α-methylpregn-4-ene-3,20-dione (Provera), adds a6α-methyl group to the basic 17α-hydroxyprogesteronestructure to greatly decrease the rate of reduction of the 4-ene-3-one system. |
General Description | Odorless white to off-white microcrystalline powder. |
Air & Water Reactions | Medroxyprogesterone 17-acetate is sensitive to prolonged exposure to air and light. Insoluble in water. |
Reactivity Profile | Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. |
Hazard | Possible carcinogen. |
Fire Hazard | Flash point data for Medroxyprogesterone 17-acetate are not available; however, Medroxyprogesterone 17-acetate is probably combustible. |
Safety Profile | Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. |
Veterinary Drugs and | In cats, MPA has been used when either castration is ineffective or undesirable to treat sexually dimorphic behavior problems such as roaming, inter-male aggressive behavi |
Treatments | ors, spraying, mounting, etc. |
Metabolism | Among the first of these substituted 17α-acetoxyprogesterone analogues to be utilized therapeutically was medroxyprogesterone acetate, a 6α-methyl progesterone analogue. |
Medroxyprogesterone Acetate Preparation Products And Raw materials | |
|---|---|
Raw materials | Sulfuric acid-->Ethylene glycol-->Peroxyacetic acid-->METHYLMAGNESIUM BROMIDE-->Acetic anhydride |
FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.