
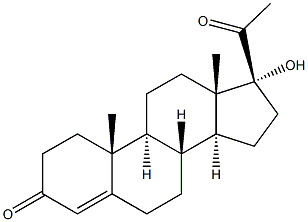
Product Name: | Hydroxyprogesterone |
|---|---|
Synonyms: | 17-Hydroxypregn-4-en-3,20-dione; 4-PREGNEN-17-OL-3,20-DIONE delta(4)-pregnene-17alpha-ol-3,20-dione |
CAS: | 68-96-2 |
MF: | C21H30O3 |
MW: | 330.46 |
EINECS: | 200-699-4 |
Product Categories: | Inhibitors; Intermediates & Fine Chemicals; |
Mol File: | 68-96-2.mol |

Hydroxyprogesterone Chemical Properties | |
|---|---|
Melting point | 276°C |
Boiling point | 407.89°C (rough estimate) |
density | 1.0998 (rough estimate) |
refractive index | 90 ° (C=1, CHCl3) |
Fp | 9℃ |
storage temp | -20C Freezer |
pka | 13.03±0.60(Predicted) |
form | neat |
Water Solubility | 5.056mg/L(20 ºC) |
Merck | 14,4839 |
BRN | 3218109 |
CAS DataBase Reference | 68-96-2(CAS DataBase Reference) |
NIST Chemistry Reference | Pregn-4-ene-3,20-dione, 17-hydroxy-(68-96-2) |
Safety Information | |
|---|---|
Hazard Codes | T.F |
Risk Statements | 61-39/23/24/25-23/24/25-11 |
Safety Statements | 53-22-36/37/39-45-36/37-16 |
RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
WGK Germany | 3 |
RTECS | TU5060000 |
HS Code | 38220090 |
Hazardous Substances Data | 68-96-2(Hazardous Substances Data) |
Hydroxyprogesterone Usage And Synthesis | |
|---|---|
Chemical Properties | White Solid |
Originator | Prodox,Upjohn |
Uses | Progesteron. It was isolated from adrenal glands. |
Uses | anticoagulant |
Uses | 17α-Hydroxy Progesterone is a metabolite of Progesterone. It was isolated from adrenal glands. |
Definition | ChEBI: A 17alpha-hydroxy steroid that is the 17alpha-hydroxy derivative of progesterone. |
Indications | Hydroxyprogesterone has been used prophylactically for the 12th to 37th week of pregnancy, particularly in women who are in the high-risk category for premature delivery (e.g., those with a history of premature delivery or spontaneous abortion). |
Manufacturing Process | A suspension of 90.0 g of δ5-pregnen-3β,17α-diol-20-one in 2300 ml of 85% formic acid was shaken for 2 h at a temperature of 70C. During this time the compound partially dissolved and at the same time a new crystalline substance appeared in the solution. After cooling, the precipitate was filtered, thus giving 80.0 g of the 3-formate of δ5-pregnen-3β,17α-diol-20-one having a melting point of 204°-207°C. 5.0 g of the 3-formate of δ5-pregnen-3β,17α-diol-20-one suspended in 120 ml of acetic anhydride was treated with 1.5 g of p-toluenesulfonic acid and the mixture was stirred for 9 h at room temperature. |
Therapeutic Function | Progestin |
Hydroxyprogesterone Preparation Products And Raw materials | |
|---|---|
Raw materials | Diosgenin-->Formic acid-->Aluminium isopropoxide-->Potassium hydroxide-->Acetic anhydride-->p-Toluenesulfonic acid |
Preparation Products | 17-hydroxy-6-methylenepregn-4-ene-3,20-dione 17-acetate-->Megestrol acetate-->3,20-Dioxopregn-4-en-17-beta-yl acetate-->6-Dibromomethylene-17-hydroxypregn-4-ene-3,20-dione 17-acetate |
FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.