
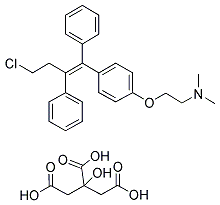
| Name | Toremifene citrate |
|---|---|
| Synonyms | (z)-4-chloro-1,2-diphenyl-1-(4-(2-(n,n-dimethylamino)ethoxy)phenyl)-1-butene;2-(4-(4-chloro-1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethyl-ethanamin(z)-ethanamin; 2-hydroxy-1,2,3-propanetricarboxylate(1:1); fareston; fc1157a; nk622;TOREMIFENE CITRATE; (z)-2-[4-(4-chloro-1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethyl-ethanamine 2-hydroxy-1,2,3-propanetricarboxylate |
| CAS NO | 89778-27-8 |
| Molecular Weight | 598.08 |
| EINECS | 663-344-6 |
| Molecular Formula | C32H36ClNO8 |
| Product Categories | XYREM; Analgesics; Active Pharmaceutical Ingredients; Amines; Aromatics; Inhibitors; Intermediates & Fine Chemicals; Pharmaceuticals |
| Mol File | 89778-27-8.mol |
| Toremifene citrate Chemical Properties | |
|---|---|
| Melting point | 160-162°C |
| form | powder |
| solubility | DMSO: >10mg/mL |
| density | 1,045g/cm |
| color | white to off-white |
| refractive index | 1,416-1,418 |
| storage temp. | -20°C Freezer |
| Merck | 14,9550 |
| Safety Information | |
|---|---|
| Hazard Codes | Xi,N,Xn |
| Risk Statements | 36/37/38-50/53-41-22 |
| Safety Statements | 26-37/39-61-60-39 |
| WGK Germany | 3 |
| RIDADR | UN 3077 9 / PGIII |
| RTECS | KH2156700 |
| Toremifene citrate Usage And Synthesis | |
|---|---|
| Chemical Properties | White-to-Off-White Solid |
| Originator | Fareston,Orion Corporation,Finland |
| Uses | An antiestrogen and antineoplastic. Nonsteroidal antiestrogen structurally similar to Tamoxifen. |
| Uses | anesthetic |
| Manufacturing Process | The reaction is performed under dry conditions. 2.1 g of lithium aluminum hydride and 50 ml of dry tetrahydrofuran are placed in a flask. Then 13.2 g of cinnamaldehyde in 50 ml of dry tetrahydrofuran are added while stirring and keeping the temperature at 25°-35°C. The stirring is continued for another 30 min at room temperature. Then 26.9 g of 4-[2-(N,N-dimethylamino)- ethoxy]benzophenone in 70 ml of dry tetrahydrofuran are added while stirring. The temperature is kept at 35°-45°C during the addition. After stirring for 2 h at 40°C the reaction mixture is poured into 150 ml of 25% ammonium chloride solution, and aluminium hydroxide is precipitated and filtered off. The filtrate is transferred to a separating funnel and the organic layer is separated. The aqueous layer is once again extracted with 60 ml of ethyl acetate. The organic layers are combined and dried over sodium sulfate. The solvent is evaporated. The residue is recrystallized from toluene. The yield is 27.5 g (68%) of 1,2-diphenyl-1-[4-[2-(N,N-dimethylamino)ethoxy]phenyl]- butane-1,4-diol. The reaction is performed under dry conditions. 40.5 g 1,2-diphenyl-1-[4-[2- (N,N-dimethylamino)ethoxy]phenyl]butane-1,4-diol and 150 ml of acetic acid anhydride are placed in a flask. The temperature is raised to 90°C, where it is kept until the primary OH-group is completely acetylated. [4-Acetoxy-1,2-diphenyl-1-[4-[2-(N,N-dimethylamino)ethoxy]phenyl]butan-1-ol is obtained as intermediate; melting point of the (RR, SS)-isomer pair is 97°-99°C. While stirring the reaction mixture, 30 ml of acetyl chloride in 50 ml of acetic acid anhydride are added at 90°C. The stirring is continued at this temperature for 2 h. The solvent is evaporated. Then 1 M sodium carbonate solution is added in excess, after which the product is extracted in toluene. The solution is dried over sodium sulfate, and the solvent is evaporated. The yield of the pure isomer mixture (Z:E 2:1) of 4-acetoxy-1,2-diphenyl-1-[4-[2-(N,N_x0002_dimethylamino)ethoxy]-phenyl]-1-butene is quantitative. The 4-acetoxy-1,2-diphenyl-1-[4-[2-(N,N-dimethylamino)ethoxy]-phenyl]-1- butene are dissolved in 94% ethanol, after which water and 20% sodium hydroxide solution are added. The mixture is refluxed for 1 h. The solution is neutralized with 2 M hydrochloric acid, after wich the ethanol is evaporated. Water is added into the residue. The product is extracted in ethyl acetate, the ethyl acetate solution is dried and the solvent is avaporated. The product is recrystallized from a mixture of water and methanol. The yield of the pure mixture of the isomers (Z:E 2:1) of 1,2-diphenyl-1-[4-[2-(N,Ndimethylamino)ethoxy]phenyl]-1-buten-4-ol, melting point 93°-100°C, is quantitative. Isolation of the (Z)-isomer as a free base: the mixture of the isomers (Z:E 2:1) of 1,2-diphenyl-1-[4-[2-(N,N-dimethylamino)ethoxy]phenyl]-1-buten-4-ol is recrystallized from toluene, and 15.9 g (41%) of the (Z)-isomer is obtained, melting point 110°-112°C. The reaction is performed under dry conditions. 42.4 g of (Z)-1,2-diphenyl-1- [4-[2-(N,N-dimethylamino)ethoxy]phenyl]-1-buten-4-ol are dissolved in 250 ml of chloroform. Then 23.8 g of thionyl chloride are added dropwise. The mixture is refluxed 3 h. The solvent is evaporated, after which the product is recrystallized from ethyl acetate. The yield of the hydrochloride salt of 4- chloro-1,2-diphenyl-1-[4-[2-(N,N-dimethylamino)ethoxy]-phenyl]-1-butene (Z) is 36.7 g (83%), melting point 194°-196°C. The base can be liberated from the salt with 1 M sodium carbonate solution, after which the base is extracted in toluene. The toluene solution is dried and the solvent is evaporated. The free base, 4-chloro-1,2-diphenyl-1-[4-[2-(N,Ndimethylamino)ethoxy]-phenyl]-1-butene (Z), has melting point 108°-110°C (from acetone). In practice it is usually used as citrate salt (1:1). |
| Brand name | Toremifene is INN and BAN. |
| Therapeutic Function | Antiestrogen, Antineoplastic |
| General Description | Toremifene, 2-[4-[(1Z)-4-chloro-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethylethanamine(Fareston), differs structurally fromtamoxifen only by having a chloroethyl group (rather than anethyl group) attached to the triphenylethylene structure. Asmight be expected, the pharmacological actions of toremifeneand tamoxifen are quite similar. Toremifene is also a SERM,with estrogen antagonist action in breast tissue but agonist actionin the endometrium, on bone tissue, and on serum lipidprofiles. Recent clinical data indicate that the incidence ofendometrial cancer is lower with toremifene use than with tamoxifen. Toremifene is used in the treatment of metastaticbreast cancer in postmenopausal women. |
| Toremifene citrate Preparation Products And Raw materials | |
|---|---|
| Raw materials |
Thionyl chloride-->Lithium Aluminum Hydride-->Ammonium chloride-->Acetyl chloride-->Acetic anhydride-->Cinnamaldehyde
|
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.