
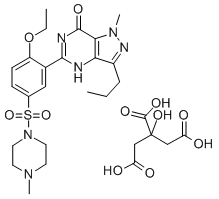
| Name | Sildenafil citrate |
|---|---|
| Synonyms | Viagra, Sildenafil citrate; 1-[[3-(4,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine citrate salt; 5-[2-Ethoxy-5-(4-methylpiperazin-1-yl)sulfonylphenyl]-1-methyl-3-propyl-4H-pyrazolo[5,4-e]pyrimidin-7-one citrate salt;1-[[3-(6,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine, 2-hydroxy-1,2,3-propanetricarboxylate; Sildenafil Citrate (100 mg);Sildenafil citrate, >=99%; Sildenafil citrate, Professional supply; 5-[2-Ethoxy-5-[(4-methyl-piperazin-1-yl)sulfonyl]phenyl]-1,6-dihydro-1-methyl-3-propyl-7H-pyrazolo[4,3-d]pyrimidin-7-one citrate 1-[[3-(4,7-DIHYDRO-1-METHYL-7-OXO-3-PROPYL-1H-PYRAZOLO[4,3-D]PYRIMIDIN-5-YL)-4-ETHOXYPHENYL]SULFONYL]-4-METHYLPIPERAZINE, CITRATE 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1h-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine citrate SILDENAFIL CITRATE VIAGRA din-5-yl)-4-ethoxyphenyl)sulfonyl)-4-methyl-,2-hydroxy-1,2,3-propanetricarbo piperazine,1-((3-(4,7-dihydro-1-methyl-7-oxo-3-propyl-1h-pyrazolo(4,3-d)pyrimi piperazine,1-((3-(4,7-dihydro-1-methyl-7-oxo-3-propyl-1h-pyrazolo(4,3-d)pyrimidin-5-yl)-4-ethoxyphenyl)sulfonyl)-4-methyl-,2-hydroxy-1,2,3-propanetricarboxylate(1:1) uk92480-10 SILDANAFIL CITRATE (BULK) SildenafilBaseC28H38N6O11S SildenafilCitrate/SildenafilBaseC28H38N6O11S 1-[[3-(4,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine, Citrate SILDENAFIL CITRATE(RG) 5(2-ETHOXY-5(4-METHYLPIPERAZINE)SULPHONYLPHENYL)-1 1-[[3-(6,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine citrate |
| CAS NO | 171599-83-0 |
| Molecular Weight | 666.7 |
| EINECS | 200-659-6 |
| Molecular Formula | C28H38N6O11S |
| Product Categories | Viagra, Revatio;Inhibitors;organic interme;Intermediates & Fine Chemicals;Pharmaceuticals;Sildenafil;Pfizer compounds; Inhibitor |
| Mol File | 171599-83-0.mol |
| Sildenafil citrate Chemical Properties | |
|---|---|
| Melting point | 187-189°C |
| form | white powder |
| Merck | 14,8489 |
| solubility | DMSO: >20mg/mL |
| Water Solubility | 3.488g/L(temperature not stated) |
| Stability | Store in Freezer |
| InChIKey | DEIYFTQMQPDXOT-UHFFFAOYSA-N |
| storage temp. | Desiccate at RT |
| CAS DataBase Reference | 171599-83-0(CAS DataBase Reference) |
| Safety Information | |
|---|---|
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 36 |
| WGK Germany | 3 |
| RTECS | TL4284390 |
| HS Code | 2933595960 |
| Sildenafil citrate Usage And Synthesis | |
|---|---|
| Chemical Properties | White Solid |
| Originator | Alsigra,Alembic Ltd.,India |
| Uses | An orally active selective type 5 cGMP phosphodiesterase inhibitor |
| Uses | agent |
| Uses | Enzyme inhibitor |
| Definition | ChEBI: The citrate salt of sildenafil. |
| Manufacturing Process | A mixture of 3-n-propylpyrazole-5-carboxylic acid ethyl ester (24.1 g, 0.132 mol) (prepared by the method of Chem. Pharm. Bull., 1984, 32, 1568) and dimethyl sulfate (16.8 g, 0.133 mol) were heated to 90°C for 2.5 h. The mixture was dissolved in dichloromethane and the solution washed with sodium carbonate solution. The organic phase was separated, dried (MgSO4) and evaporated under vacuum to give a solid. Chromatography on silica gel (300 g), eluting with dichloromethane gave the 1-methyl-3-n-propylpyrazole- 5-carboxylic acid ethyl ester as a colourless oil (20.4 g, 79%). 1-Methyl-3-n-propylpyrazole-5-carboxylic acid ethyl ester (20.2 g, 0.10 mol) was suspended in 6 N aqueous sodium hydroxide solution (50 ml, 0.30 mol). The mixture was heated to 80°C for 2 h then diluted with water (50 ml) and acidified with concentrated hydrochloric acid (25 ml). Filtration gave the 1- methyl-3-n-propylpyrazole-5-carboxylic acid as pale brown crystals (12.3 g, 71%), melting point 150°-154°C. 1-Methyl-3-n-propylpyrazole-5-carboxylic acid (12.1 g, 0.072 mol) was added portionwise to a mixture of oleum (13 ml) and fuming nitric acid (11 ml), keeping the temperature below 60°C. After the addition, the mixture was heated at 60°C overnight and then cooled to room temperature before being poured onto ice. Filtration of the precipitate gave the 1-methyl-4-nitro-3-npropylpyrazole- 5-carboxylic acid as a white solid (11.5 g, 75%), melting point 124°-127°C. 1-Methyl-4-nitro-3-n-propylpyrazole-5-carboxylic acid (11.3 g, 0.053 mol) was added to thionyl chloride (50 ml) and the resulting mixture heated under reflux for 3 h. The reaction mixture was then cooled and excess thionyl chloride removed by evaporation under vacuum. The oily residue was dissolved in acetone (50 ml) and the solution cautiously added to a mixture of ice (50 g) and concentrated aqueous ammonium hydroxide solution (50 ml). The precipitate was collected by filtration to provide the 1-methyl-4-nitro-3-npropylpyrazole- 5-carboxamide as a pale yellow solid (8.77 g, 78%), melting point 141°-143°C. 1-Methyl-4-nito-3-n-propylpyrazole-5-carboxamide (3.45 g, 16.2 mmol) and stannous chloride dihydrate (18.4 g, 81 mmol) were suspended in ethanol and the mixture heated under reflux for 2 h. The resulting solution was cooled to room temperature, basified to pH 9 by the addition of 2 N aqueous sodium hydroxide solution and extracted with dichloromethane (3 x 150 ml). The organic extracts were combined, dried (MgSO4) and evaporated under vacuum. Trituration of the residue with ether gave the 4-amino-1-methyl-3-npropylpyrazole- 5-carboxamide as an off-white solid (2.77 g, 94%), melting point 98°-101°C. A solution of 2-ethoxybenzoyl chloride (6.1 g, 33.0 mmol) in dichloromethane (50 ml) was added to a stirred solution of 4-amino-1-methyl-3-npropylpyrazole- 5-carboxamide (3.0 g, 16.4 mmol), 4-dimethylaminopyridine (0.02 g, 0.1 64 mmol) and triethylamine (3.34 g, 33.0 mmol) in dichloromethane (50 ml) at 0°C. The resulting mixture was allowed to warm to room temperature and stirred for a further 2 h. The solvent was evaporated under vacuum, the residue dissolved in a 19:1 mixture of dichloromethane and methanol (250 ml), and then the solution washed with 1 N hydrochloric acid (100 ml), dried (MgSO4) and evaporated under vacuum. The crude material was chromatographed on silica gel (200 g), eluting with a 97:3 mixture of dichloromethane and methanol, to give a pink solid; crystallisation from ethyl acetate-hexane gave the 4-(2-ethoxybenzamido)-1-methyl-3-npropylpyrazole- 5-carboxamide as a pale pink solid (2.2 g, 40%), melting point 153°-155°C. 4-(2-Ethoxybenzamido)-1-methyl-3-n-propylpyrazole-5-carboxamide (223 g, 0.676 mol) was added portionwise to a solution of sodium hydroxide (54 g, 1.35 mol) and 30% hydrogen peroxide solution (224 ml) in water (2000 ml). Ethanol (700 ml) was added and the resulting mixture heated under reflux for 2.5 h, cooled, then evaporated under vacuum. The resulting solid was treated with 2 N hydrochloric acid (380 ml), with external cooling, and the mixture was extracted with dichloromethane (1 x 700 ml, 3 x 200 ml). The combined organic extracts were washed successively with saturated aqueous sodium carbonate solution (3 x 400 ml) and brine (300 ml), then dried (Na2SO4) and evaporated under vacuum. Chromatography of the residue on silica gel (1000 g), using a methanol in dichloromethane elution gradient (0-1%), followed by trituration of the crude product with ether (300 ml), gave the 5-(2- ethoxyphenyl)-1-methyl-3-n-propyl-l,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin- 7-one as a colourless solid (152.2 g, 72%), melting point 143°-146°C. 5-(2-Ethoxyphenyl)-1-methyl-3-n-propyl-l,6-dihydro-7H-pyrazolo[4,3- d]pyrimidin-7-one (10.0 g, 32.1 mmol) was added portionwise to chlorosulfonic acid (20 ml) at 0°C under a nitrogen atmosphere. After being stirred overnight, the reaction solution was cautiously added to ice-water (150ml) and the aqueous mixture extracted with a 9:1 mixture of dichloromethane and methanol (4 x 100 ml). The combined extracts were dried (Na2SO4) and evaporated under vacuum to give the required 5-(5-chlorosulphonyl-2- ethoxyphenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin- 7-one as a white solid (12.8 g, 97%), melting point 179°-181°C. 4-Methylpiperidine was added to a stirred suspension of 5-(5-chlorosulphonyl- 2-ethoxyphenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3- d]pyrimidin-7-one in ethanol at room temperature. The resulting mixture was stirred for 4 days before removing the solvent by evaporation under vacuum. The residue was dissolved in a 9:1 mixture of dichloromethane and methanol and the solution washed with saturated aqueous sodium carbonate solution. The aqueous phase was further extracted with dichloromethane-methanol mixtures (3 x 100 ml) and all the organic fractions were combined, dried (MgSO4) and evaporated under vacuum to give a solid. Crystallisation from a mixture of methanol-dimethylformamide gave the 5-[2-ethoxy-5-(4- methylpiperidinylsulphonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7Hpyrazolo[ 4,3-d]-pyrimidin-7-one as an off-white solid, melting point 187°- 189°C. After addition of citric acid to the 5-[2-ethoxy-5-(4- methylpiperidinylsulphonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7Hpyrazolo[ 4,3-d]-pyrimidin-7-one (sildenafil) the it's salt is obtained, namely sildenafil citrate. |
| Brand name | Viagra (Pfizer). |
| structure and hydrogen bonding | Sildenafil citrate (SC) has been widely used for the treatment of erectile disorder. A detailed study concerning solid-state structure of this compound is very important for understanding enzyme (PDE5)-inhibitor (sildenafil) interaction. It is also of interest to determine sildenafil’s protonation sites, as they may be responsible for its binding to the phosphodiesterase acidic amino acids. Sildenafil citrate (Viagra) and sildenafil base in pure form were characterized by 1H, 13C, 15N NMR spectroscopy in solution, solid-state, and pharmaceutical dosage forms.42 The analysis of chemical shifts showed that: (i) N6-H forms intramolecular hydrogen bonds, (ii) N25 is protonated in the salt, and (iii) intermolecular OH. . .N hydrogen bonds involving N2 and N4 are present in the solid sildenafil citrate. The 13C CPMAS spectra of the tablets containing different amounts of sildenafil citrate were recorded and showed that chemical shifts of sildenafil citrate in pure form and in pharmaceutical dosage forms are the same. SC is easily detected in the pharmaceutical dosage forms since only two of its carbon resonances (OCH2 and quaternary carbon of the citrate anion) fall into carbohydrate-type region of the excipient. Solid-state 13C and 15N MAS NMR have recently been used to investigate how water interacts with SC.43 When the humidity is altered, the water concentration in the solid compound changes but does not reach a stoichiometric (e.g., 1:1) ratio to form a true hydrate. Only one set of 15N and 13C signals was observed for each humidity level indicating that water incorporated into the crystal lattice of SC is very mobile and exchanges rapidly between various sites. The 13C data showed the formation of a hydrogen bond between water molecule and one carbonyl of the citrate anion. The spectra also show that the water content affects the conformation of the propyl group. Additionally, 15N dipolar dephasing (DD) experiments confirmed that the sildenafil molecule is only protonated in the piperazine ring. |
| Therapeutic Function | Vasodilator |
| Sildenafil citrate Preparation Products And Raw materials | |
|---|---|
| Raw materials |
|
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.