
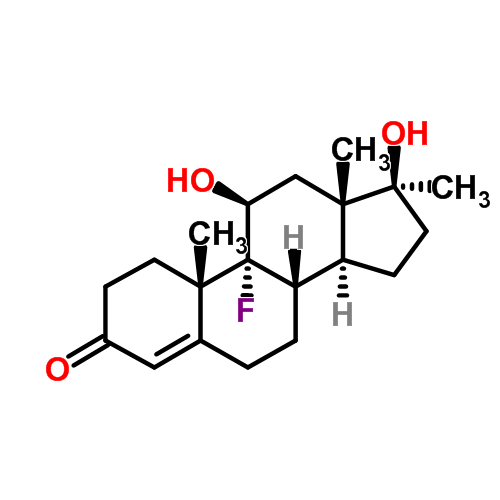
| Name | Fluoxymesterone |
|---|---|
CAS NO | 76-43-7 |
EINECS | 200-961-8 |
Molecular Formula | C20H29FO3 |
Molecular Weight | 336.4409 |
Other Name | 9-alpha-fluoro-11-beta,17-beta-dihydroxy-17-alpha-methylandrost-4-en-3-one (11beta,17beta)-9-fluoro-11,17-dihydroxy-17-methylandrost-4-en-3-one fluoxymesterone standard solution fluoxymesterone—dea schedule iii 11BETA,17BETA-DIHYDROXY-9ALPHA-FLUORO-17ALPHA-METHYL-4-ANDROSTEN-3-ONE 4-ANDROSTEN-9-ALPHA-FLUORO-17-ALPHA-METHYL-11-BETA, 17-BETA-DIOL-3-ONE 9a-fluoro-11b,17b-dihydroxy-17a-methyl-4-androsten-3-one 9ALPHA-FLUORO-11BETA-HYDROXY-17ALPHA-METHYLTESTOSTERONE FLUOXYMESTERONE 11-beta,17-beta-Dihydroxy-9-alpha-fluoro-17-alpha-methyl-4-androster-3-one 17-alpha-methyl-9-alpha-fluoro-11-beta-hydroxytesterone 17-alpha-Methyl-9-alpha-fluoro-11-beta-hydroxytestosterone 9-alpha-fluoro-11-beta,17-beta-dihydroxy-17-alpha-methyl-4-androstene-3-one 9alpha-Fluoro-11beta,17beta-dihydroxy-17alpha-methyl-4-androstene-3-one 9-alpha-fluoro-11-beta-hydroxy-17-methyltestosterone 9alpha-Fluoro-11beta-hydroxy-17-methyltestosterone 9-alpha-Fluoro-17-alpha-methyl-11-beta,17-dihydroxy-4-androsten-3-one 9-fluoro-11,17-dihydroxy-17-methyl-,(11-beta,17-beta)-androst-4-en-3-on 9-fluoro-11,17-dihydroxy-17-methyl-,(11beta,17beta)-androst-4-en-3-on 9-Fluoro-11,17-dihydroxy-17-methylandrost-4-en-3-one 9-fluoro-11-beta,17-beta-dihydroxy-17-methyl-androst-4-en-3-on 9-fluoro-11beta,17beta-dihydroxy-17-methyl-androst-4-en-3-on 9-Fluoro-11-beta,17-beta-dihydroxy-17-methylandrost-4-en-3-one Androfluorene |
Density | 1.22g/cm3 |
| Melting Point(℃) | 240℃ |
| Boiling Point | 474.2°C at 760 mmHg |
| Refractive Index | 1.562 |
| Flash Point | 240.6°C |
| Vapour Pressure | 5.5E-11mmHg at 25°C |

Fluoxymesterone Chemical Properties | |
|---|---|
Melting point | 240 °C |
alpha | 104 º (c=1,EtOH) |
Boiling point | 474.2±45.0 °C(Predicted) |
density | 1.0455 (estimate) |
storage temp. | 20°C |
solubility | H2O: ≤0.5 mg/mL |
pka | 13.40±0.70(Predicted) |
form | solid (photosensitive) |
color | white |
Water Solubility | NEGLIGIBLE |
Merck | 13,4212 |
CAS DataBase Reference | 76-43-7(CAS DataBase Reference) |
NIST Chemistry Reference | 4-Androsten-3-one, 9alpha-fluoro-11beta,17beta-dihydroxy-17alpha-methyl-,(76-43-7) |
EPA Substance Registry System | Fluoxymesterone (76-43-7) |
Safety Information | |
|---|---|
Hazard Codes | Xn,T,F |
Risk Statements | 63-38-19-11-61-60 |
Safety Statements | 22-36-24/25-45-53 |
WGK Germany | 3 |
RTECS | BV8390000 |
HS Code | 29372900 |
Hazardous Substances Data | 76-43-7(Hazardous Substances Data) |
Toxicity | LD50 intraperitoneal in mouse: 2350mg/kg |
Fluoxymesterone Usage And Synthesis | |
|---|---|
Chemical Properties | white to light yellow crystal powde |
Originator | Halotestin, Upjohn, US,1957 |
Uses | Fluoxymesterone is an anabolic steroid with androgenic activity. |
Uses | stimulates erythropoesis and cell respiration treatment of male hypogonadismdelayed puberty in males, managing metastatic breast cancer in menopausal women |
Manufacturing Process | The following description is taken from US Patent 2,793,218. |
Brand name | Android (Valeant); |
Therapeutic Function | Androgen |
General Description | Fluoxymesterone, 9α-fluoro-11β,17β-dihydroxy-17-methylandrost-4-en-3-one, is ahighly potent, orally active androgen, about 5 to 10 timesmore potent than testosterone. |
Pharmacokinetics | By substituting a 9α-fluoro group onto an analog of 17α-methyltestosterone, fluoxymesterone has 20 times the anabolic and 10 times the androgenic activity of 17α-methyltestosterone. |
| Safety Profile | Poison by ingestion. |
FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.