
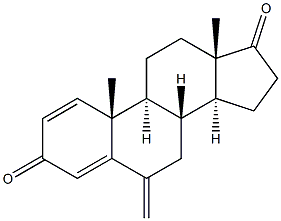
| Product Name | Exemestane |
|---|---|
| Synonyms | ExeMestane (USP); ExeMestane(AroMasin); 1,4-Androstadien-3,17-dione-6-Methylene-d2; (8R,9S,10R,13S,14S)-10,13-Dimethyl-6-methylene-7,8,9,11,12,13,15,16-octahydro-6H-cyclopenta[a]phenanthrene-3,17(10H,14H)-dione; ExeMestine; (8R,9S,10R,13S,14S)-10,13-diMethyl-6-Methylene-7,8,9,10,11,12,13,14,15,16-decahydro-3H-cyclopenta[a]phenanthrene-3,17(6H)-dione; ExeMestane SynonyMs 6-Methylenandrosta-1,4-diene-3,17-dione;PNU155971 10,13-dimethyl-6-methylidene-7,8,9,10,11,12,13,14,15,16-decahydrocyclopenta[a]phenanthrene- 6-methylenandrosta-1,4-diene-3,17-dione 6-METHYLENEANDROSTA-1,4-DIENE-3,17-DIONE AROMASIN EXEMESTANE FCE-24304 Exemestan Exemestance 6-Methyleneandrosta-1,4-diene-3,17-dione, FCE-24304, Aromasin Androsta-1,4-diene-3,17-dione, 6-methylene EXALAMIDE |
| CAS | 107868-30-4 |
| MF | C20H24O2 |
| MW | 296.4 |
| EINECS | 643-090-2 |
| Product Categories | Intermediates & Fine Chemicals; Pharmaceuticals; Steroids; Pfizer compounds; Steroid and Hormone; API; Inhibitors; Anti-cancer&immunity; Active Pharmaceutical Ingredients; chiral; Antineoplastic (Hormonal) |
| Mol File | 107868-30-4.mol |
| Exemestane Chemical Properties | |
|---|---|
| Melting point | 155.13°C |
| Boiling point | 453.7±45.0 °C(Predicted) |
| density | 1.13±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility DMSO | ≥20mg/mL |
| form | powder |
| color | white to off-white |
| optical activity | [α]/D +250 to +300°, c = 1 in methanol |
| InChIKey | BFYIZQONLCFLEV-DAELLWKTSA-N |
| CAS DataBase Reference | 107868-30-4(CAS DataBase Reference) |
| Safety Information | |
|---|---|
| Hazard Codes | T,N |
| Risk Statements | 60-61-51 |
| Safety Statements | 53-22-36/37-57 |
| WGK Germany | 3 |
| HS Code | 29372900 |
| Hazardous Substances Data | 107868-30-4(Hazardous Substances Data) |
| Exemestane Usage And Synthesis | |
|---|---|
| Indications and Usage | Exemestane is an irreversible steroid aromatase inhibitor. Its structure is similar to that of aromatase’s natural substrate, androstenedione, and acts as a pseudosubstrate. Postmenopausal women’s estrogen is mainly converted from androgen (produced by the adrenal cortex) by aromatase in the surrounding tissue. This drug irreversibly binds with the active site on aromatase to deactivate it, thus dramatically lower estrogen levels in the blood circulation of postmenopausal women. By inhibiting aromatase to lower estrogen levels, it can be used to treat hormone-dependent breast cancer in postmenopausal women. Exemestane is suitable for treating advanced breast cancer in naturally or artificially postmenopausal women that has not responded well to tamoxifen treatment. It is also suitable for treating estrogen and progesterone receptor positive postmenopausal advanced breast cancer, and it can also be used to treat metastasized breast cancer and as adjuvant therapy for early breast cancer. |
| Pharmacokinetics | This drug has no noticeable effect on adrenal corticosteroids biosynthesis. Even when its concentration is over 600 times the concentration required to inhibit aromatase, it still has no noticeable effect the other enzymes in the corticosteroid production pathway. This drug is absorbed quickly when taken orally and will affect food absorption. Its oral bioavailability is 42%. Postmenopausal women have a higher absorption rate than healthy test subjects. Patients reach peak blood concentration 2-4 hours after intake, and the peak lasts for an average of 1.2 hours, which is 2.9 hours shorter than healthy subjects. It mainly binds to Α1-acid glycoprotein and protein, and its overall binding rate to protein is 90%. It is mainly metabolized by the liver, the metabolite is inactive 17-Hydrecoxetron, and its clearing half-life is 24 hours. It is mostly excreted through urine and feces, which both account for 42% of the consumed amount. |
| Drug interactions | 1. This drug cannot be used in combination with estrogen-based drugs to avoid counteracting its medicinal effects. 2. Exemestane is mostly metabolized by CYP3A4, but when used in combination with a strong CYP3A4 inhibitor (Ketoconazole), its pharmacokinetics do not exhibit any change. This is because the inhibitor does not seem to affect the drug’s pharmacokinetics, but it is also possible that the known CYP3A4 inducer lowers the blood concentration of Exemestane. |
| Side effects | Adverse effects include nausea, dryness, constipation, diarrhea, dizziness, insomnia, rash, fatigue, fever, swelling, pain, vomiting, abdominal pain, increased appetite, weight gain, etc. Additionally, some literature reports cases of hypertension, depression, anxiety, difficulty breathing, coughing, etc. There may also be decreases lymph cell amounts, abnormalities in liver function indicators (such as alanine aminotransferase), etc. |
| Clinical Research | For patients resistant to tamoxifen, 25mg of Exemestane, qd, can achieve an objective efficacy rate of 15-28% and a median continuation period of 69-76 weeks. Exemestane is superior to Megestrol, and it can extend the disease progression time. For patients who have worsened conditions following Megestrol treatment, Exemestane can still achieve an objective efficacy rate of 11-13%. A daily 25mg dose of Exemestane has an objective efficacy rate of 6.6% on patients who have not responded to non-steroidal aromatase inhibitors, and the two drugs are not cross-resistant. |
| Contradictions | 1. Not to be used by patients allergic to this drug. 2. Not to be used by pregnant women, breastfeeding women, and children. |
| Warnings and precautions | 1. Premenopausal women usually do not use Exemestane. 2. Patients with moderate to severe liver or renal failure should use with caution. Exceeding the recommended dosage of Exemestane may increase the occurrence of nonfatal adverse effects. 3. FDA labeled this drug’s pregnancy safety as level D. 4. Elderly patients do not require any special precautions. |
| Description | Exemestane was launched in US and other countries for the treatment of estrogendependent tumors and postmenopausal breast cancer. It is a novel steroidal compound structurally related to the natural substrate for the biosynthesis of estrogen, androstanedione, and can be synthesized by methylidenation of androsta-1, 4-dien- 17beta-ol-3-one in 6 position then oxidation of the alcohol function. Exemestane is an irreversible inactivator of the aromatase enzyme system, so inducing in vivo a dose-related sustained suppression of serum estrogen and minimal endocrine activity. It is the first steroidal representative of the third-generation of orally active aromatase inhibitors with a highly potent and selective mechanism of action, displaying good tolerability and safety profile. In rats with DMBA-induced mammary tumors, 10 to 100 mglkg of exemestan administered po twice-daily for 4 weeks resulted in 76 to 88% regression. In women failing anti-estrogen therapy with tamoxifen, this agent has demonstrated high activity in locally advanced or metastatic disease. In addition, it may also have potential for breast cancer prevention. |
| Chemical Properties | white to light yellow crystal powder |
| Originator | Farmitalia Carlo Erba (Italy) |
| Uses | An antineoplastic (hormonal) |
| Uses | antiinfective |
| Uses | Labeled Exemestane, intended for use as an internal standard for the quantification of Exemestane by GC- or LC-mass spectrometry. |
| Definition | ChEBI: A 17-oxo steroid that is androsta-1,4-diene-3,17-dione in which the hydrogens at position 6 are replaced by a double bond to a methylene group. A selective inhibitor of the aromatase (oestrogen synthase) system, it is used in the treatment of advanced brea t cancer. |
| Manufacturing Process | 0.50 g of 6-methylenandrost-4-ene-3,17-dione and 0.57 g of dichlorodicyanobenzoquinone were refluxed in 20 ml of anhydrous dioxane for about 15 hours. To remove the DDQ the suspension was filtered through alumina. After evaporation of the solvent the residue was dissolved in ethyl acetate, the organic layer washed with water, dried over sodium sulfate and the solvent removed under vacuum. The crude product was chromatographed on silica gel using hexane/ethyl acetate to yield 0.25 g of pure 6- methylenandrosta-1,4-diene-3,17-dione, m.p. 188-191°C, λmax 247 nm (ε 13.750). |
| Brand name | Aromasin (Pharmacia& Upjohn). |
| Therapeutic Function | Antineoplastic |
| General Description | Exemestane, 6-methylenandrosta-1,4-diene-3,17-dione (Aromasin), is the first steroid-basedaromatase inhibitor approved for the treatment of breastcancer in the United States. It is a mechanism-based inactivatorthat irreversibly inhibits the enzyme. Plasma estrogenlevels are reduced by 85% to 95% within 2 to 3 days, and effectslast 4 to 5 days. Exemestane does not inhibit any of themajor cytochromes P450 and has essentially no interactionwith steroid receptors, with only a very weak affinity for theAR. The 17β-hydroxyexemestane reduction product, however,has much higher affinity for the AR than the parent(still several fold less than DHT, 0.28% for parent vs. 30%for metabolite). The clinical significance of the affinity islikely minimal because of the low levels of the metaboliteproduced. |
| Hazard | A reproductive hazard. |
| Exemestane Preparation Products And Raw materials | |
|---|---|
| Raw materials | Ethanol-->Tetrahydrofuran-->Formaldehyde-->p-Toluenesulfonic acid-->Benzoic acid-->2,3-Dichloro-5,6-dicyano-1,4-benzoquinone-->19-Norandrost-5(10)-ene-3,17-dione |
FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.