
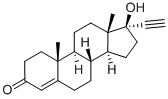
Product Name: | Ethisterone |
|---|---|
Synonyms: | Prone ETHISTERONE |
CAS: | 434-03-7 |
MF: | C21H28O2 |
MW: | 312.45 |
EINECS: | 207-096-5 |
Product Categories: | Steroids; |
Mol File: | 434-03-7.mol |

Ethisterone Chemical Properties | |
|---|---|
Melting point | 263-269 °C(lit.) |
alpha | D23 +23.8° (dioxane); D25 -32.0° (pyridine) |
Boiling point | 392.36°C (rough estimate) |
density | 1.0697 (rough estimate) |
refractive index | 33 ° (C=1, Pyridine) |
storage temp. | -20°C Freezer |
pka | 13.10±0.60(Predicted) |
form | neat |
Water Solubility | soluble in chloroform, DMSO (0.05 mg/ml), acetone (slightly ), ethanol (<1 mg/ml at 25°C), and water (<1 mg/ml at 25°C). |
Merck | 14,3741 |
BRN | 1889895 |
InChIKey | CHNXZKVNWQUJIB-CEGNMAFCSA-N |
CAS DataBase Reference | 434-03-7 |
NIST Chemistry Reference | Ethisterone(434-03-7) |
Safety Information | |
|---|---|
Hazard Codes | Xn,T |
Risk Statements | 40-48-61 |
Safety Statements | 22-24/25-45-53 |
RIDADR | UN 2811 |
WGK Germany | 3 |
RTECS | TU5570250 |
HS Code | 29372390 |
Ethisterone Usage And Synthesis | |
|---|---|
Chemical Properties | Off-White Powder |
Originator | Lutocyclin,Ciba |
Uses | Synthetic progestogen; metabolite of Danazol; intermediate in the synthesis of Spironolactone |
Definition | ChEBI: A 17beta-hydroxy steroid that is testosterone in which the 17beta hydrogen is replaced by an ethynyl group. |
Manufacturing Process | 0.5 part of δ5:6-17-ethinyl-androstendiol-(3:17) is dissolved in 10 parts of dry acetone, the solution is mixed with a solution of 1 part of tertiary aluminum butylate in 40 parts of absolute toluene and the whole is heated to boiling in a reflux apparatus for 21 hours. |
Therapeutic Function | Progestin |
Ethisterone Preparation Products And Raw materials | |
|---|---|
Raw materials | Dehydroepiandrosterone |

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.