
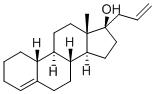
Product Name: | Allylestrenol |
|---|---|
Synonyms: | 13-methyl-17-prop-2-enyl-2,3,6,7,8,9,10,11,12,14,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ol 17-hydroxy-17-alpha-allyl-4-estrene |
CAS: | 432-60-0 |
MF: | C21H32O |
MW: | 300.48 |
EINECS: | 207-082-9 |
Product Categories: | Steroid and Hormone; Inhibitors; Medicine intermediate |
Mol File: | 432-60-0.mol |

Allylestrenol Chemical Properties | |
|---|---|
Melting point | 79.5-80° |
Boiling point | 381.7°C (rough estimate) |
density | 0.9914 (rough estimate) |
refractive index | 1.4800 (estimate) |
pka | 14.95±0.40(Predicted) |
Merck | 14,291 |
CAS DataBase Reference | 432-60-0(CAS DataBase Reference) |
Safety Information | |
|---|---|
RTECS | KG7960000 |
Allylestrenol Usage And Synthesis | |
|---|---|
Originator | Alilestrenol ,Terapia |
Uses | A synthetic steroid with progestational activity. |
Manufacturing Process | To 145 ml of dry methylamine which is cooled to -20°C 1.5 g of lithium cut to small pieces are added. Then the reaction mixture is poured into acidified ice water. |
Therapeutic Function | Progestin; Antiandrogen |
Allylestrenol Preparation Products And Raw materials | |
|---|---|
Raw materials | Lithium-->Magnesium-->Acetic acid-->Allyl bromide-->Chromium(VI) oxide-->Methylamine |

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.