Progesterone Usage And Synthesis |
|---|
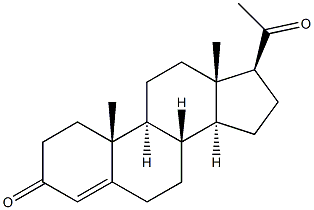
Description | Progesterone is a hormone released by luteal cells in the ovaries which contains 21 Carbon Atoms.
Progesterone is also a crucial metabolic intermediate in the production of other endogenous steroids.
There are two crystal forms of progesterone, that are type-α and type-β, the two types have similar physiological activity.
Type-α is precipitated from dilute ethanol as orthorhombic white prismatic crystal, while type-β is orthorhombic white needle crystal, they are both insoluble in water, but soluble in ethanol, ethyl ether, chloroform, acetone, dioxane and concentrated sulfuric acid.
Progesterone can be released by the ovary, placenta and adrenal cortex. Its physiological function mainly manifests in promoting estrogen treated over thicken lining of the uterus to continue the development, proliferation and thickening and hypertrophy, soften and secretion of mucus in order to make good condition for implantation of the fertilized egg.
After the implantation, early stage survival and development of fertilized egg is also under the control of the high progesterone release.
As a result, progesterone is a very important hormone in the female reproductive system, and it is also an important intermediate in the biosynthesis of steroid hormones.
All steroid hormone releasable glands can produce progesterone, but only ovarian and placenta can release progesterone as the main hormone.
Ovarian will release large amount of progesterone in the luteal phase after ovulation by granulose luteal cells, so progesterone is also named as progestin. Progesterone will decrease in result of corpus luteum atrophy.
For normal women, placenta will becomes the main organ maintain progesterone after 8 to 9 weeks of pregnancy, accompany with the ovary releasing until the end of pregnancy. |
Uses | The main physiological effects of progesterone:
1. Progesterone can maintain the female animal pregnancy, and cause a series of physiological changes, such as inhibition of female estrus.
2. Progesterone has the power to promote the thickening of the lining of the uterus, promote the bending of the gland and to increase secretion function.
3. Progesterone can inhibit the peristalsis of the uterus, and contribute to the cervix contraction, secretion of mucus, etc.. These physiological changes provide suitable environment for the operation, growth and development of early embryos, as well as the continued growth of the fetus.
4. Small amount of progesterone is also used in combination with the hormone estrogen to promote female estrus. The synergy between progesterone and prolactin can promote the development of mammary glands.
5. Progesterone is involved in the feedback regulation of the hypothalamus and anterior pituitary, which makes the balance of the animal reproductive hormones. In vivo, progesterone content of all sorts of livestock follicular phase is below 1 ng/ml, while in bovine corpus luteum period is approximately 4 ng/ml, pregnancy period is about 18 ng/ml.
6. Formerly biochemical study shows that progesterone modulates action as progestogens, clinical for the treatment of habitual abortion, dysmenorrhea, amenorrhea and other symptoms. One of progesterone's most important functions is as hormone drugs, to promote and maintain the uterine changes in the early stage of pregnancy, used in habitual abortion, irregular menstruation, etc.. In addition, progesterone also behaves as steroid hormone drug as well as progestogens, which is used in treatment of threatened abortion. |
Preparation | Progesterone can be obtained by oxidation of the pregnenolone.
Dry toluene was added to a oven dried reaction kettle, and then cyclohexanone and pregnenolone were added in order with vigorous stirring to dissolve.
Side product H2O was removed by Soxhlet extraction with toluene steam, aluminium isopropoxidequickly was added flowingly, the oxidation reaction was hold on at 115 oC for 2h, cooling to 80 oC, add 5% dilute sulfuric acid under stirring then stand by until water and toluene separated, the toluene layer was extracted with water to neutrality and then distillation off toluene and cyclohexanone.
Cooling, filtering, filter cake was beated with petroleum, filtering, washing with petroleum, dried as crude progesterone.
The crude product was dissolved in ethanol, decolorized by activated carbon, recrystallized to get the final product, yield 80%. Another way to produce progesterone is choosing the 16-Dehydropregnenolone acetate as start material, treated consecutively by catalytic hydrogenation, alkali hydrolysis, oxidation by aluminum isopropoxide, to get the progesterone as final product. |
Chemical Properties | White powder.
Melting point 121°C.
Stable in air.
Insoluble in water.
A female sex hormone.
Low toxicity. |
Occurrence | Colchicum luteum also yields this alkaloid. |
Uses | Sterid hormone produced by the corpus luteum.
Induces maturation and secretory activity of the uterine endothelium; suppresses ovulation.
Progesterone is implicated in the etiology of breast cancer |
Definition | progesterone: hormone, producedprimarily by the corpus luteumof the ovary but also by theplacenta, that prepares the inner liningof the uterus for implantation ofa fertilized egg cell.
If implantationfails, the corpus luteum degeneratesand progesterone production ceasesaccordingly.
If implantation occurs,the corpus luteum continues to secreteprogesterone, under the influenceof luteinizing hormone andprolactin, for several months of pregnancy,by which time the placentahas taken over this function.
Duringpregnancy, progesterone maintainsthe constitution of the uterus andprevents further release of eggs fromthe ovary.
Small amounts of progesteroneare produced by the testes. |
Definition | ChEBI: A C21-steroid hormone in which a pregnane skeleton carries oxo substituents at positions 3 and 20 and is unsaturated at C(4)-C(5). As a hormone, it is involved in the female menstrual cycle, pregnancy and embryogenesis of humans and ot er species. |
Brand name | Crinone (Columbia); Prometrium (Unimed). |
General Description | Progesterone, pregn-4-en-3,20-dione, is so rapidly metabolized that it is not particularlyeffective orally, being only one twelfth as active as intramuscularly.An oral formulation of micronized progesterone(Prometrium) is available.
Progesterone given intramuscularlycan be very irritating.
A vaginal gel containing 4% or8% progesterone offers an alternative dosage form. Progesterone was originally obtained from animal ovariesbut is now prepared synthetically from plant sterol precursors.
The discovery of 19-nortestosterones with progesteroneactivity made synthetically modified progestins of tremendoustherapeutic importance.
Progesterone (and all other steroid 4-ene-3-ones) is lightsensitive and should be protected from light. |
Air & Water Reactions | Insoluble in water. |
Reactivity Profile | Progesterone is sensitive to light . |
Hazard | A carcinogen (OSHA). |
Health Hazard | ACUTE/CHRONIC HAZARDS: Progesterone may be absorbed through the skin. |
Fire Hazard | Flash point data for Progesterone are not available; however, Progesterone is probably combustible. |
Biological Activity | Endogenous progesterone receptor (PR) agonist (EC 50 = 0.5 nM). |
Safety Profile | NTP 10th Report on Carcinogens.
IARC Cancer Review: Animal Lirmted Evidence IMEMDT 21,491,79;
Animal Sufficient Evidence IMEMDT 6,135,74. EPA Genetic Toxicology Program.
Reported in EPA TSCA Inventory.
SAFETY PROFILE: Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data.
Poison by intravenous and intraperitoneal routes.
Human teratogenic effects by ingestion and parenteral routes: developmental abnormalities of the urogenital system.
Human male reproductive effects by intramuscular route: changes in spermatogenesis, the prostate, seminal vesicle, Cowper's gland and accessory glands, impotence, and breast development.
Human female reproductive effects by ingestion, parenteral, and intravaginal routes: ferthty changes; menstrual cycle changes and disorders; uterus, cervix, and vagina changes.
Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. |
Carcinogenicity | Progesterone is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. |