
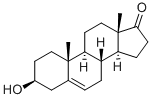
Name | Estradiol |
|---|---|
CAS | 50-28-2 |
Synonyms | 17BETA-ESTRADIOL-16,16,17-D3 BETA-ESTRADIOL-16,16,17-D3 (+)-3,17β-estradiol (17b)-estra-1,3,5(10)-triene-3,17diol (17β)-estra-1,3,5(10)-triene-3,17diol .alpha.-Oestradiol .beta.-Oestradiol 17-.beta.-Estra-1,3,5(10)-triene-3,17-diol 17-.beta.-Oestra-1,3,5(10)-triene-3,17-diol 17-beta-estra-1,3,5(10)-triene-3,17-diol 17beta-Estra-1,3,5(10)-triene-3,17-diol 17-beta-oestra-1,3,5(10)-triene-3,17-diol 17beta-Oestra-1,3,5(10)-triene-3,17-diol 17-beta-oh-estradiol 17beta-OH-estradiol 17-beta-oh-oestradiol 17beta-OH-oestradiol 17β-estradiol 3,17-.beta.-Dihydroxy-1,3,5(10)-oestratriene 3,17-.beta.-Dihydroxyestra-1,3,5(10)-triene CAS: 50-28-2 MF: C18H24O2 MW: 272.38 EINECS: 200-023-8 Melting point:178-179 °C(lit.) Alpha: D25 +76 to +83° (dioxane) Boiling point: 355.44°C (rough estimate) Density: 1.0708 (rough estimate) Refractive index: 80.4 ° (C=1, Dioxane) Flash point:2℃ Storage temp: 2-8°C Solubility: Practically insoluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent), slightly soluble in methylene chloride. Pka: pKa 10.71±0.02(H2O(0.1% p-dioxane) t=25±0.1 I=0.03(KCl))(Approximate) Form: powder Color: White to off-white Water Solubility: Soluble in dimethyl sulfoxide, ethanol , water, phosphate buffer saline, dimethyl formamide, acetone, dioxane and alkali hydroxides. Slightly soluble in vegetable oils. Merck 14,3703 BRN 1914275 Stability: Stable. Incompatible with strong oxidizing agents. |

Safety Information | |
|---|---|
Hazard Codes | T,Xn,F |
Risk Statements | 60-61-45-63-64-40-36-20/21/22-11-48 |
Safety Statements | 53-22-36/37/39-45-36/37-26-16-36-20 |
RIDADR | 2811 |
WGK Germany | 3 |
RTECS | KG2975000 |
F | 8-10 |
HazardClass | 6.1 |
PackingGroup | III |
HS Code | 29372390 |
Hazardous Substances Data | 50-28-2(Hazardous Substances Data) |
Toxicity | LD50 subcutaneous in rat: > 300mg/kg |
β-ESTRADIOL Usage And Synthesis | |
|---|---|
Indications and Usage | Estradiol is a white or milky white ordorless crystalline powder. Estradiol is the intermediate between estradiol valerate and estradiol benzoate, and it is a type of estrogen drug. |
Adverse reactions | In high dosages, estradiol can inhibit the release of anterior pituitary prolactin, thus decreasing breast milk secretion. |
Contradictions | Do not use on breasts, vaginal area and vaginal mucosa. |
Chemical Properties | White or almost white, crystalline powder or colourless crystals. |
Chemical Properties | Estradiol, 17-β-is an odorless white to yellow crystalline substance. |
Uses | 17β-Estradiol is the major estrogen secreted by the premenopausal ovary. |
Uses | Estradiol is the major estrogen secreted by the premenopausal ovary. |
Uses | Estradiol USP (Estrace) is used to treat Breast cancer; prostatic carcinoma. |
Definition | ChEBI: The 17beta-isomer of estradiol. |
Acquired resistance | Estradiol is the most potent endogenous estrogen, exhibiting high affinity for the ER and high potency when administered parenterally. |
General Description | Estradiol, estra-1,3,5(10)-triene-3,17β-diol, is the most activeof the natural steroid estrogens. A new transdermal spray, Evamist, was approvedin 2007. Estradiol 17-valerate, USP (IM injection) |
Hazard | A carcinogen (OSHA). |
Biological Activity | Endogenous estrogen receptor (ER) agonist (K i values are 0.12 and 0.13 nM for ER α and ER β respectively). |
Contact allergens | Natural estradiol, used in transdermal systems for hormonal substitution, can induce allergic contact dermatitis, with the risk of systemic contact dermatitis after oral reintroduction. |
Safety Profile | Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. |
Potential Exposure | The working environment may be contaminated during sex hormone manufacture, especially during the extraction and purification of natural steroid hormones; grinding of raw materials; handling of powdered products and recrystallization. |
Shipping | UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials |
Purification Methods | 17-Estradiol (previously known as -estradiol) is purified by chromatography on SiO2 (toluene/EtOAc 4:1) and recrystallised from CHCl3/hexane or 80% EtOH. |
β-ESTRADIOL Preparation Products And Raw materials | |
|---|---|
Raw materials | Nitrogen-->p-Toluenesulfonic acid-->Lithium-->Potassium borohydride-->Biphenyl-->Acetylacetone-->1,3,5(10)-Estratrien-3-ol-17-one-->Diphenylmethane-->4-ETHYL-2-METHYLPYRIDINE-->ADD |

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.