
| Name | Eplerenone |
|---|---|
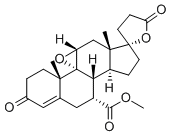
| Synonyms | plerenone; (2'R,4aS,4bR,5aR,6aS,9aS,9bR,10R)-Methyl 4a,6a-diMethyl-2,4'-dioxo-3,4,4a,4',5a,5',6,6a,8,9,9a,9b,10,11-tetradecahydro-2H,3'H-spiro[cyclopenta[1,2]phenanthro[4,4a-b]oxirene-7,2'-furan]-10-carboxylate; Eplerenone [USAN]; HSDB 7522; Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, gamma-lactone, methyl ester, (7alpha,11alpha,17alpha)-;Eplerenone intermediate Eplerenone; (7α,11α,17α)-9,11-epoxy-17-hydroxy-3-oxo-γ-lactone-pregn-4-ene-7,21-dicarboxylic acid-7-methyl ester; Eplerenone for system suitability Inspra SC-66110 CGP 30083 HSDB 7522 EPLERENONE EPLERINONE Elperenone epoxymexrenone EPLERENONE 98% Eplerenone [USAN] Eplerenone-13C-d3 EplerenoneC24H3006 EPLERENONE USP STANDARD γ-Lactone 7-Methyl Ester EPLERENONE AND N-1 INTERMEDIATE Eplerenone for system suitability Eplerenone for peak identification Eplerenone intermediate Eplerenone (7α,11α,17α)-9,11-Epoxy-17-hydroxy-3-oxopregn-4-ene-7,21-dicarboxylic Acid pregn-4-ene-7,21-dicarboxylic acid 9,11-epoxy-17-hydroxy-3-oxo gamma-lactone methyl ester (7α,11α,17α)-9,11-Epoxy-17-hydroxy-3-oxo-pregn-4-ene-7,21-dicarboxylicacidγ-lactonemethylester (7a,11a,17a)-9,11-Epoxy-17-hydroxy-3-oxopregn-4-ene-7,21-dicarboxylic Acid -Lactone 7-Methyl Ester (7A,11A,17A)-9,11-EPOXY-17-HYDROXY-3-OXO-PREGN-4-ENE-7,21-DICARBOXYLIC ACID G-LACTONE METHYL ESTER (7α,11α,17α)-9,11-epoxy-17-hydroxy-3-oxo-γ-lactone-pregn-4-ene-7,21-dicarboxylic acid-7-methyl ester pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo, γ-lactone, methyl ester (7 α, 11 α, 17 α) 9,11alpha-Epoxy-17-hydroxy-3-oxo-17alpha-pregn-4-ene-7alpha,21-dicarboxylic Acid gamma-Lactone Methyl Ester Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, gamma-lactone, methyl ester, (7alpha,11alpha,17alpha)- CGP 30083, Epoxymexrenone, Inspra, Spiro[9,11-epoxy-9H-cyclopenta[a]phenanthrene-17(2H),2(3-furan],pregn-4-ene-7,21-dicarboxylic Acid, Inspra, Methyl (1'R,2R,2'S,9'R,10'S,11'S,15'S,17'R)-2',15'-diMethyl-5,5'-dioxo-18'-oxaspiro[oxolane-2,14'-pentacyclo[8.8.0.0^{1,17}.0^{2,7}.0^{11,15}]octadecan]-6'-ene-9'-carboxylate (2'R,4aS,4bR,5aR,6aS,9aS,9bR,10R)-Methyl 4a,6a-diMethyl-2,4'-dioxo-3,4,4a,4',5a,5',6,6a,8,9,9a,9b,10,11-tetradecahydro-2H,3'H-spiro[cyclopenta[1,2]phenanthro[4,4a-b]oxirene-7,2'-furan]-10-carboxylate |
| CAS NO | 107724-20-9 |
| EINECS | 600-850-8 |
| Molecular Weight | 414.49 |
| Molecular Formula | C24H30O6 |
| Product Categories | Cardiovascular APIs;Inspra;Antihypertensive;Steroid and Hormone;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Steroids |
| Mol File | 107724-20-9.mol |
Eplerenone Chemical Properties | |
|---|---|
| Melting point | 241-243°C |
| alpha | D +5° (c = 0.437 in chloroform) |
| Boiling point | 597.9±50.0 °C(Predicted) |
| density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | Store at RT |
| solubility | DMSO: soluble2mg/mL, clear (warmed) |
| form | powder |
| color | white to beige |
| λmax | 240nm(lit.) |
| Merck | 14,3625 |
| InChIKey | UZZAHAKTLYRDOK-MGIDJNSKSA-N |
| Safety Information | |
|---|---|
| RIDADR | 3077 |
| WGK Germany | 3 |
| HS Code | 29322090 |
| Hazardous Substances Data | 107724-20-9(Hazardous Substances Data) |
| Eplerenone Usage And Synthesis | |
|---|---|
| Description | Eplerenone is used alone or in combination with other medications to treat high blood pressure. Eplerenone is in a class of medications called mineralocorticoid receptor antagonists. It works by blocking the action of aldosterone, a natural substance in the body that raises blood pressure. |
| Pharmacodynamics | Eplerenone, an aldosterone receptor antagonist similar to spironolactone, has been shown to produce sustained increases in plasma renin and serum aldosterone, consistent with inhibition of the negative regulatory feedback of aldosterone on renin secretion. The resulting increased plasma renin activity and aldosterone circulating levels do not overcome the effects of eplerenone. Eplerenone selectively binds to recombinant human mineralocorticoid receptors relative to its binding to recombinant human glucocorticoid, progesterone and androgen receptors. |
| Biological activity | Eplerenone belongs to a class of drugs called aldosterone antagonists. A class of drugs is a group of medications that work in a similar way. These drugs are often used to treat similar conditions. Eplerenone works by interfering with the activity of a steroid in your body called aldosterone. Aldosterone acts to increase the amount of sodium and water you retain. This increased sodium and water can cause high blood pressure, which can in turn cause heart failure. |
| Interactions | Eplerenone is primarily metabolized by the cytochrome P450 enzyme CYP3A4. Thus the potential exists for adverse drug interactions with other drugs that induce or inhibit CYP3A4. Specifically, the concomitant use of the CYP3A4 potent inhibitors ketoconazole and itraconazole is contraindicated. Other CYP3A4 inhibitors including erythromycin, saquinavir, and verapamil should be used with caution. Other drugs that increase potassium concentrations may increase the risk of hyperkalemia associated with eplerenone therapy, including salt substitutes, potassium supplements and other potassium-sparing diuretics. |
| Overview | Eplerenone is in a class of medications called mineralocorticoid receptor antagonists. It can be used individually or in combination with other medications to treat hypertension by blocking the action of aldosterone, a natural substance in the body that raises blood pressure. |
| Chemical properties | Eplerenone is an odorless, white to off-white crystalline powder. It is very slightly soluble in water, with its solubility essentially pH-independent. The octanol/water partition coefficient of eplerenone is approximately 7.1 at pH 7.0. |
| Mechanism of action | Aldosterone,with many physiological and pathological effects, can cause central blood pressure and endothelial injury (catecholamines enhance its role), reduce heart rate variability, induce ventricular arrhythmias, and promote retention of sodium, potassium and magnesium loss, promote myocardial fibrosis, necrosis and inflammation, damage the fibrinolytic system. Angiotensin converting enzyme inhibitors (also called angiotensin converting enzyme inhibitors, referred to as ACEI) and angiotensin Ⅱ receptor antagonist ARB aldosterone can inhibit the secretion of adrenaline, but after a period of treatment. The release of aldosterone was restored, which may even exceed the baseline plasma concentration levels. Despite adequate treatment of ACEI and ARB, aldosterone-induced damage can still happen, so it is necessary to use aldosterone receptor antagonists in the treatment of hypertension. Clinical studies have shown that patients who are not satisfied with the efficacy of ACEI or ARB therapy can add eplerenone along with the treatment. Non-selective aldosterone receptor antagonist spironolactone can reduce mortality in patients with congestive heart failure, However, the side effects of male hyperplasia and other diseases associated with sex hormones have limited its application in the treatment of hypertension. |
| Adverse effects | Common adverse drug reactions (ADRs) associated with the use of eplerenone include: hyperkalaemia, hypotension, dizziness, altered renal function, and increased creatinine concentration. |
| References | https://en.wikipedia.org/wiki/Eplerenone https://medlineplus.gov/druginfo/meds/a603004.html http://www.rxlist.com/inspra-drug.htm |
| Description | Eplerenone derives its antihypertensive effect by blocking the binding of aldosterone at the mineralocorticoid receptor (MR). The drug, which was previously approved only for the oral treatment of hypertension, is now indicated to improve survival of stable patients with left ventricular systolic dysfunction (ejection fraction <40%) and clinical evidence congestive heart failure (CHF) after an acute myocardial infarction. Aldosterone is a key hormone in the renin-angiotensin-aldosterone system (RAAS), which is of critical importance in the development and progression of hypertension, cardiac remodeling and other cardiovascular diseases. The purpose of RAAS is to control sodium, potassium, and fluid volume balance. Aldosterone binds to MRs in both epithelial (e.g. kidney) and nonepithelial (e.g. heart, blood vessels, and brain) tissues and increases blood pressure through induction of sodium reabsorption and possibly other mechanisms. The actions of aldosterone can be blocked by spironolactone (Aldactone ?), a relatively nonselective MR antagonist that has been used in clinical practice for many years. Eplerenone, a structural analog of spironolactone, is a highly selective MR antagonist, with significantly lower affinity for other nuclear receptors. It can be prepared by several related ways, with the key step being the introduction of 11-a-hydroxy group on the steroid scaffold via microbiological conversion. The presence of the 11-a-hydroxy group permits the derivation of the epoxy functionality found in eplerenone. Following oral administration, eplerenone is well absorbed and reaches peak plasma concentrations in~2 h. The bioavailability of eplerenone is 98% and it is cleared predominantly by CYP3A4 metabolism, with an elimination half-life of 4–6 h. Steady state is reached within two days. Eplerenone therapy is typically initiated with 25 mg once daily oral dosing and, if tolerated by the patient, titrated to 50 mg once daily. In a clinical study, eplerenone significantly reduced deaths in congestive heart failure patients after a heart attack, above and beyond standard therapy, including ACE inhibitors and β-blockers. The trial in more than 6600 hospitalized patients demonstrated a 15% reduction in the risk of death for eplerenone compared with placebo, in addition to standard treatment. The most commonly reported adverse events associated with eplerenone are hyperkalemia and increased creatine. |
| Chemical Properties | White Solid |
| Originator | Ciba-Geigy (Novartis) (US) |
| Uses | Selective aldosterone receptor antagonist (SARA), structurally similar to Spiranolactone. Eplerenone is used alone or in combination with other medications to treat high blood pressure. Eplerenone is in a class of medications called mineralocorticoid receptor antagonists. It works by blocking the action of aldosterone, a natural substance in the body that raises blood pressure. |
| Uses | Eplerenone is an aldosterone antagonist with an IC50 of 0.36 μM. It is used as an adjunct in the management of chronic heart failure. It is similar to the diuretic spironolactone, though it may be more specific for the mineralocorticoid receptor and is sp |
| Uses | anticancer agent |
| Brand name | Inspra (Searle). |
| General Description | Eplerenone, 9,11α-epoxy-17α-hydroxy-3-oxopregn-4-ene-7α,21-dicarboxylic acid, γ-lactone,methyl ester (Inspra), is a newer aldosterone antagonist that isused for the treatment of hypertension. |
| Biological Activity | Selective mineralocorticoid (aldosterone) receptor antagonist (IC 50 = 360 nM). Displays > 27-fold selectivity over androgen, progesterone and estrogen receptors (IC 50 > 10 μ M). Orally active antihypertensive in vivo |
| Clinical Use | A newer drug, eplerenone, has a structure similar to that of spironolactone and a similar mechanism of action. It was initially approved for use in the treatment of hypertension but it can now be used in the treatment of patients with left ventricular systolic dysfunction and congestive heart failure after myocardial infarction. |
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.