
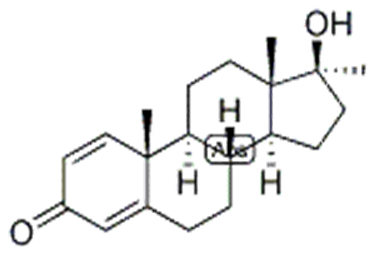
| Name |
|
|---|---|
| Synonyms | 1,2-Dehydro-17-methyltestosterone 17-alpha-methyl-17-beta-hydroxy-1,4-androstadien-3-one 17alpha-methyl-17beta-hydroxyandrosta-1,4-dien-3-one 17alpha-Methyl-1-dehydrotestosterone 17beta-Hydroxy-17alpha-methylandrosta-1,4-dien-3-one 17-beta-hydroxy-17-alpha-methyl-androsta-4-dien-3-one 17-beta-Hydroxy-17-alpha-methylandrostra-1,4-dien-3-one 17-beta-hydroxy-17-methyl-androsta-4-dien-3-one 17beta-hydroxy-17-methyl-androsta-4-dien-3-one 17-hydroxy-17-methyl-4-dien-3-on(17-beta)-androsta- 17-hydroxy-17-methyl-4-dien-3-on(17beta)-androsta- 17-Hydroxy-17-methylandrosta-1,4-dien-3-one 17-Methyl-17-hydroxy-1,4-androstadi-en-3-one 1-Dehydro-17-methyltestosterone a1-dehydromethyltesterone Abirol Anabolicum Medivet Anabolin Andoredan Androsta-1,4-dien-3-one, 17beta-hydroxy-17alpha-methyl- |
| CAS NO | 72-63-9 |
| EINECS | 200-787-2 |
| Molecular Weight | 300.44 |
| Molecular Formula | C20H28O2 |
| Product Categories | Finished Steroid and Hormone; Intermediates & Fine Chemicals; Pharmaceuticals; Steroid and Hormone; API; Biochemistry; Hydroxyketosteroids; Steroids |
| Mol File | Mol File |
Metandienone Chemical Properties | |
|---|---|
| density | 1.0597 (rough estimate) |
| Boiling point | 381.66°C (rough estimate) |
| Fp | -2℃ |
| form | neat |
| Melting point | 165-166 °C |
| refractive index | 1.4800 (estimate) |
| storage temp | 2-8°C |
| pka | 15.12±0.60(Predicted) |
| Merck | 5951 |
| BRN | 2056255 |
| optical activity | [α]1.15/D 0.5±0.5° in chloroform |
| InChIKey | XWALNWXLMVGSFR-HLXURNFRSA-N |
| CAS DataBase Reference | 72-63-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Methandrostenolone(72-63-9) |
| Safety Information | |
|---|---|
| Hazard Codes | F,T |
| Risk Statements | 60-61-11-19-38 |
| Safety Statements | 53-45 |
| WGK Germany | 3 |
| RTECS | BV8000000 |
| HS Code | 29329970 |
| Toxicity | LD50 oral in rat: > 1gm/kg |
| Hazardous Substances Data | 72-63-9(Hazardous Substances Data) |
| F | 8 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Metandienone Usage And Synthesis | |
|---|---|
| Indications and Usage | Metandienone is a dehydrogenation derivative of methyltestosterone. Its protein assimilating effects are similar to those of testosterone propionate, but its androgen effects are slightly weaker and are about 1/100 those of the latter. This drug can promote protein synthesis, inhibit protein heterogeneity, maintain a positive nitrogen balance, improve appetite, aid muscle growth, and encourage weight gain. It can cause calcium and phosphorous deposition in bone tissue, promote bone mesenchymal cell formation, and increase bone calcification and growth. It can encourage tissue generation and granulation and speed up healing of wounds. It lowers blood cholesterol and improves fat metabolism. Metandienone is an androgen and anabolic hormone drug. It is suitable for people with insufficient protein synthesis and increased protein decomposition, such as patients with negative nitrogen balance caused by chronic wasting diseases, severe infections, trauma, and burns, as well as malignant tumors, osteoporosis, stunted children, dwarfism, fractures, less healing, and high cholesterol. |
| Drug Interactions | When Metandienone is used with oxyphenbutazone, the blood concentration of oxyphenbutazone may increase. |
| Adverse reactions | 1. Metandienone’s adverse are identical to those of regular androgen and anabolic steroids. There may be nausea, vomiting, indigestion, diarrhea, and other digestive tract reactions. Long term, high dosage use may lead to bone weight, water-sodium retention, increased blood supply to skin, hypocalcemia, hyperosteogeny, etc. Women may experience slight virilism, with effects including acne, hirsuitism, lowered voice, clitoral hypertrophy, irregular menstruation, etc. 2. Metandienone’s side effects on the liver include jaundice and liver failure. There have been reports of liver cancer and benign hepatocellular adenoma tied to Metandianone use. |
| Contradictions | 1. Do not use this drug if allergic. 2. Do not use if experiencing liver failure. 3. Do not use during pregnancy or if pregnancy may occur during treatment. 4. Do not use if experiencing prostate cancer, kidney disease, hypertension, or prostate hypertrophy. |
| Warnings and Precautions |
1. Monitor liver functions while using Metandienone. |
| Chemical Properties | White Solid |
| Originator | Dianabol,Ciba,US,1960 |
| Uses | Anabolic steroid. Androgen. Controlled substance. |
Manufacturing Process | As described in US Patent 2,929,763, methandrostenolone may be made by a fermentation route. 2 g of sodium nitrate, 1 g of primary potassium orthophosphate, 0.5 g of magnesium sulfate heptahydrate, 0.5 g of potassium chloride, 50 g of glucose and 1 g of Difco yeast extract are dissolved in one liter of tap water, brought to pH 5 by addition of a sodium hydroxide solution and sterilized. The resulting nutrient solution is inoculated with 50 cc of a 4- day-old shaking culture of Didymella lycopersici and shaken for 48 hours at 27°C, whereby the culture becomes well developed. To two liters of a culture so prepared there is added under sterile conditions a solution of 500 mg of 17α-methyl-testosterone in 15 cc of acetone. Shaking is carried out for 3 days at 27°C, the mycellium then filtered off with suction, washed with water and ethyl acetate and the combined filtrates extracted with ethyl acetate. The extraction residue obtained after evaporation of the solvent is dissolved in a little acetone. On addition of ether, the 1-dehydro-17αmethyl-testosterone is obtained in compact crystals. MP 163° to 164°C. An alternative synthetic route is described in US Patent 2,900,398 as follows. A suspension of 30 g of 17α-methyl-testosterone and 10 g of selenium dioxide in 600 cc of tertiary amyl alcohol is treated with 60 g of magnesium powder and 6 cc of glacial acetic acid. The mixture is refluxed for 24 hours with good stirring in an atmosphere of nitrogen, another 10 g of selenium dioxide being added after 10 hours. After some cooling, the suspension is filtered through some Hyflo and washed thoroughly with ethyl acetate. The resulting brown solution is evaporated in vacuo and the residue dissolved in ethyl acetate. The ethyl acetate solution is then washed with water, dried and evaporated. To remove any selenium still present, the residue is dissolved in 200 cc of methanol and mixed with 100 g of iron powder and 2 g of active carbon. The mixture is heated for 30 minutes with stirring under reflux, then filtered with suction, washed with methanol and the solution evaporated in vacuo. The residue is then chromatographed on 900 g of aluminum oxide. The residues of the evaporated benzene and ether fractions are treated with active carbon in methanol or acetone, evaporated again, and the residue recrystallized from a mixture of acetone and ether. There are obtained 17.5 g of pure 1-dehydro17α-methyl-testosterone which melts at 163° to 164°C. |
| Therapeutic Function | Androgen, Anabolic |
<
/table>
| Metandienone Preparation Products And Raw materials | |
|---|---|
| Raw materials | Selenium dioxide |
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.