
| Name |
|
|---|---|
| Synonyms | Prone |
| CAS NO | 434-03-7 |
| EINECS | 207-096-5 |
| Molecular Weight | 312.45 |
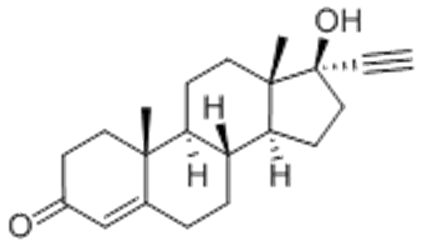
| Molecular Formula | C21H28O2 |
| Product Categories | Steroids; Acetylenes; Biochemistry; Functionalized Acetylenes; Hydroxyketosteroids; Intermediates & Fine Chemicals; Metabolites & Impurities; Inhibitors; progestogen estrogen; Metabolites & Impurities, Pharmaceuticals, Intermediates & Fine Chemicals; Steroid and Hormone; Pharmaceuticals |
| Mol File | 434-03-7.mol |
| Ethisterone Chemical Properties | |
|---|---|
| density | 1.0697 (rough estimate) |
| Boiling point | 392.36°C (rough estimate) |
| form | neat |
| Melting point | 263-269 °C(lit.) |
| alpha | D23 +23.8° (dioxane); D25 -32.0° (pyridine) |
| refractive index | 33 ° (C=1, Pyridine) |
| storage temp | -20°C Freezer |
| pka | 13.10±0.60(Predicted) |
| Water Solubility | soluble in chloroform, DMSO (0.05 mg/ml), acetone |
| Merck | 14,3741 |
| BRN | 1889895 |
| InChIKey | CHNXZKVNWQUJIB-CEGNMAFCSA-N |
| CAS DataBase Reference | 434-03-7 |
| NIST Chemistry Reference | Ethisterone(434-03-7) |
| Safety Information | |
|---|---|
| Hazard Codes | Xn,T |
| Risk Statements | 40-48-61 |
| Safety Statements | 22-24/25-45-53 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | TU5570250 |
| HS Code | 29372390 |
| Ethisterone Usage And Synthesis | |
|---|---|
| Chemical Properties | Off-White Powder |
| Originator | Lutocyclin,Ciba |
| Uses | Synthetic progestogen; metabolite of Danazol; intermediate in the synthesis of Spironolactone |
| Definition | ChEBI: A 17beta-hydroxy steroid that is testosterone in which the 17beta hydrogen is replaced by an ethynyl group. Ethisterone was the first orally active progestin and is a metabolite of danazol. |
| Manufacturing Process | 0.5 part of δ5:6-17-ethinyl-androstendiol-(3:17) is dissolved in 10 parts of dry acetone, the solution is mixed with a solution of 1 part of tertiary aluminum butylate in 40 parts of absolute toluene and the whole is heated to boiling in a reflux apparatus for 21 hours. After the reaction mixture has cooled it is diluted with 100 parts of ether, the solution is washed with dilute mineral acid and with water, dried and the solvent is evaporated. In this manner there is obtained δ5:6-17-ethinyl-androstene-3-one-17-ol (ethisterone); MP: 270°- 272°C; it may be recrystallized from ethyl acetate. |
| Therapeutic Function | Progestin |
| Ethisterone Usage And Synthesis | |
|---|---|
| Chemical Properties | Off-White Powder |
| Originator | Lutocyclin,Ciba |
| Uses | Synthetic progestogen; metabolite of Danazol; intermediate in the synthesis of Spironolactone |
| Definition | ChEBI: A 17beta-hydroxy steroid that is testosterone in which the 17beta hydrogen is replaced by an ethynyl group. Ethisterone was the first orally active progestin and is a metabolite of danazol. |
| Manufacturing Process | 0.5 part of δ5:6-17-ethinyl-androstendiol-(3:17) is dissolved in 10 parts of dry acetone, the solution is mixed with a solution of 1 part of tertiary aluminum butylate in 40 parts of absolute toluene and the whole is heated to boiling in a reflux apparatus for 21 hours. After the reaction mixture has cooled it is diluted with 100 parts of ether, the solution is washed with dilute mineral acid and with water, dried and the solvent is evaporated. In this manner there is obtained δ5:6-17-ethinyl-androstene-3-one-17-ol (ethisterone); MP: 270°- 272°C; it may be recrystallized from ethyl acetate. |
| Therapeutic Function | Progestin |
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.