
| Name |
|
|---|---|
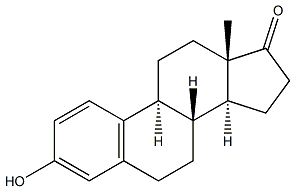
| Synonyms | 1,3,5(10)-ESTRATRIEN-3-OL-17-ONE 1,3,5[10]-ESTRATRIENE-3-OL-17-ONE 1,3,5-ESTRATRIEN-3-OL-17-ONE 3beta-hydroxyestra-1,3,5(10)-trien-17-one 3-HYDROXY-1,3,5[10]-ESTRATRIEN-17-ONE 3-HYDROXY-ESTRA-1,3,5(10)-TRIEN-17-ONE beta-estrone DELTA1,3,5(10)-ESTRATRIEN-3-OL-17-ONE DESTRONE E 1 ESTROL ESTRONE FOLLICULIN KESTRONE OESTRIN OESTRONE OESTRONE (E1) (8R,13S)-3-Hydroxy-13-methyl-6,7,8,9,11,12,13,14,15,16-decahydro-cyclopenta[a]phenanthren-17-one 1,3,5(10)-Oestratrien-3-ol-17-one 1,3,5-Oestratrien-3-ol-17-one |
| CAS NO | 53-16-7 |
| EINECS | 200-164-5 |
| Molecular Weight | 270.37 |
| Molecular Formula | C18H22O2 |
| Melting point | 258-260 °C(lit.) |
| Alpha | 158 º (c=1, dioxane) |
| Boiling point | 353.48°C (rough estimate) |
| Density | 1.2360 |
| Refractive index | 165 ° (C=1, Dioxane) |
| Flash point | 9℃ |
| Storage temp | Refrigerator |
| Pka | pKa 10.77±0.02(H2O)(Approximate) |
| Form | Crystalline Powder or Crystals |
| Color | White to almost white |
| Water Solubility | 0.03 g/L |
| Merck | 3708 |
| BRN | 1915077 |
| Safety Information | |
|---|---|
| Hazard Codes | T,F |
| Risk Statements | 45-60-61-64-40-63-39/23/24/25-23/24/25-11 |
| Safety Statements | 53-45-36/37-16 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | KG8575000 |
| Hazardous Substances Data | 53-16-7(Hazardous Substances Data) |
| HS Code | 29335995 |
| 1,3,5(10)-Estratrien-3-ol-17-one Usage And Synthesis | |
|---|---|
| Chemical Properties | Crystalline Solid |
| Chemical Properties | Estrone is an odorless white crystalline powder. |
| Originator | Estrone,Abbott |
| Uses | Estrone is a metabolite of 17β-Estradiol (E888000). During the metabolism, it is in rapid equilibrium with Estriol (E888960) and 17β-Estradiol (E888000) (1). Causes the feminization of male fish at human and animal waste sites (2). This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants. |
| Uses | A metabolite of 17β-Estradiol. |
| Uses | estrogen |
| Definition | ChEBI: A 17-oxo steroid that is estra-1,3,5(10)-triene substituted by an hydroxy group at position 3 and an oxo group at position 17. |
| Manufacturing Process | 1-Vinyl-1,2,3,4-tetrahydronaphthalene-1,6-diol reacts with 2- methylcyclopentane-1,3-dione in the presence of Triton B in tert-butanol gives a good yield of δ1,3,5(10),9(11)-8,14-secoestratetraen-3-ol-14,17-dione, melting point 124°-126°C (from methanol). δ1,3,5(10),9(11)-8,14-Secoestratetraen-3-ol-14,17-dione under influence of hydrochloric acid in tetrahydrofurane cyclises into δ1,3,5(10),8,14-estrapentaen- 3-ol-17-one, melting point 216°-218°C. δ1,3,5(10),8,14-Estrapentaen-3-ol-17-one is converted to d,l-8-dehydroestrone by selective hydrogenation with hydrogen, melting point 251°-254°C (from methanol). Exhaustive hydrogenation of δ1,3,5(10),8,14-estrapentaen-3-ol-17- one give d,l-8-isoestrone. d,l-8-Isoestrone in the presence of hydrochloric acid in tetrahydrofurane isomerizes into d,l-9(11)-dehydroestrone, melting point 262°-265°C (from alcohol). Hydrogenation of d,l-9(11)-dehydroestrone in tetrahydrofuran in the presence of Pd/CaCO3 yields the estrone, melting point 251°-252°C (from acetone). |
| Brand name | Estrogenic Substance (Wyeth); Theelin (Parkdale) |
| Therapeutic Function | Estrogen |
| General Description | Estrone, 3-hydroxyestra-1,3,5(10)-trien-17-one, is less active than estradiol but more active than itsmetabolite, estriol. As the salt of its 3-sulfate ester, estroneis the primary ingredient in conjugated estrogens, USP, andesterified estrogens, USP. Although originally obtainedfrom the urine of pregnant mares (about 10 mg/L), estroneis now prepared synthetically. Estrone itself is not availablein commercial oral formulations, but can be obtained at compounding pharmacies as a topical formulation. Oleoylestrone,the C3 ester of estrone with oleic acid, is in phase IIclinical trials for the treatment of obesity. This acyl estronederivative reduces fat stores by a mechanism not involvingthe ER, although some of the oleoyl-estrone is hydrolyzedto estrone in vivo. |
| Hazard | A carcinogen (OSHA) |
| Safety Profile | Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. A poison by intraperitoneal and subcutaneous routes. Human reproductive effects by implantation: spermatogenesis and impotence. Mutation data reported. A steroid drug for the treatment of menopause and ovariectomy symptoms. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Potential Exposure | Synthesized from ergosterol. Used in combination with progestogen as an oral contraceptive. |
| Shipping | UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials. |
| Purification Methods | Purify estrone by chromatography on silica gel, eluting with 2:1 hexane/EtOAc and recrystallising from EtOH or Et2O/EtOH. [Danishefsky & Cain J Am Chem Soc 98 4975 1976.] The acetate [901-93-9] crystallises from EtOH with m 125-127o. [Beilstein 8 III 1171.] |
| Incompatibilities | May react exothermically with reducing agents to generate flammable gaseous hydrogen. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. |
| 1,3,5(10)-Estratrien-3-ol-17-one Preparation Products And Raw materials | |
|---|---|
| Raw materials | Isopropyl acetate-->Acetylacetone-->CHLORIC ACID-->2,4,6-Collidine-->5-ALPHA-ANDROSTANE-->Hydrogen-->Hydrochloric acid-->Palladium-->Bis(4-hydroxyphenyl) Sulfone-->Benzyltrimethylammonium hydroxide-->19-DIOL 3-ACETATE |
| Preparation Products | Oxendolone-->β-ESTRADIOL-->16,17-Epoxy-3,17-dihydroxyestra-1,3,5(10)-triene-3,17-diacetate-->Ethynyl estradiol-->estra-1,3,5(10),16-tetraene-3,17-diol diacetate-->Estriol |
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.