
| Name | Prednisolone |
|---|---|
| Synonyms | DEHYDROHYDROCORTISONE; HYDRODELTALONE; METACORT AND RALONE; METACORTANDROLONE; 11B,17,21-TRIHYDROXYPREGNA-1,4-DIENE-3,20-DIONE; 11BETA,17ALPHA,21-TRIHYDROXYPREGNA-1,4-DIENE-3,20-DIONE; 11BETA 17,21-TRIHYDROXYPREGNA-1,4-DIENE-3,20-DIONE; 11-BETA,17-ALPHA,21-HYDROXY-1,4-PREGNADIENE-3,20-DIONE 11B,17,21-TRIHYDROXYPREGNA-1,4-DIENE-3,20-DIONE 11BETA 17,21-TRIHYDROXYPREGNA-1,4-DIENE-3,20-DIONE 11-BETA,17-ALPHA,21-HYDROXY-1,4-PREGNADIENE-3,20-DIONE 11BETA,17ALPHA,21-TRIHYDROXY-1,4-PREGNADIENE-3,20-DIONE 11BETA,17ALPHA,21-TRIHYDROXYPREGNA-1,4-DIENE-3,20-DIONE 1,4-PREGNADIEN-11-B,17,21-TRIOL-3,20-DIONE 1,4-PREGNADIEN-11-BETA, 17,21-TRIOL-3,20-DIONE 1,4-PREGNADIEN-11BETA,17ALPHA,21-TRIOL-3,20-DIONE 1,4-PREGNADIENE-11B,17A,21-TRIOL-3,20-DIONE 1,4-PREGNADIENE-11BETA,17ALPHA,21-TRIOL-3,20-DIONE 1-DEHYDROCORTISOL 1-DEHYDROHYDROCORTISONE (8S,9S,10R,11S,13S,14S,17R)-11,17-DIHYDROXY-17-(2-HYDROXY-ACETYL)-10,13-DIMETHYL-6,7,8,9,10,11,12,13,14,15,16,17-DODECAHYDRO-CYCLOPENTA[A]PHENANTHREN-3-ONE DEHYDROHYDROCORTISONE HYDRODELTALONE METACORT AND RALONE METACORTANDROLONE PREDNISOLONE PRENOLONE SCHERISOLON |
| CAS NO | 50-24-8 |
| EINECS | 200-021-7 |
| Molecular Weight | 360.44 |
| Molecular Formula | C21H28O5 |
| Product Categories | API; DELTA-CORTEF; Steroid and Hormone; Intermediates & Fine Chemicals; Pharmaceuticals; Steroids & Hormones - 13C & 2H; Antitumors for Research and Experimental Use; Biochemistry; Hydroxyketosteroids; Steroids |
| Mol File | 50-24-8.mol |
Prednisolone Chemical Properties | |
|---|---|
| Melting point | 240 °C (dec.)(lit.) |
| density | 1.0963 (rough estimate) |
| Boiling point | 412.46°C (rough estimate) |
| refractive index | 100 ° (C=1, Dioxane) |
| storage temp | -20°C Freezer |
| solubility | Very slightly soluble in water, soluble in ethanol (96 per cent) and in methanol, sparingly soluble in acetone, slightly soluble in methylene chloride. It shows polymorphism (5.9). |
| form | powder |
| alpha | D25 +102° (dioxane) |
| BRN | 1354103 |
| Water Solubility | 2.225g/L(25 ºC) |
| Merck | 14,7721 |
| CAS DataBase Reference | 50-24-8(CAS DataBase Reference) |
| InChIKey | OIGNJSKKLXVSLS-VWUMJDOOSA-N |
| EPA Substance Registry System | Prednisolone (50-24-8) |
| Safety Information | |
|---|---|
| Toxicity | LD50 oral in mouse: 1680mg/kg |
| Hazardous Substances Data | 50-24-8(Hazardous Substances Data) |
| Prednisolone Usage And Synthesis | |
|---|---|
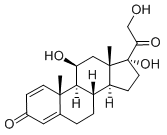
| Overview | Prednisolone is a synthetic adrenocortical steroid drug with predominantly glucocorticoid properties[1, 2, 4, 10]. Prednisone is a white to practically white, odorless, crystalline powder and has a molecular weight of 358.43. Prednisone is very slightly soluble in water, slightly soluble in alcohol, chloroform, dioxane, and methanol[3]. Some of these properties reproduce the physiological actions of endogenous glucocorticosteroids, but others do not necessarily reflect any of the adrenal hormones' normal functions; they are seen only after administration of large therapeutic doses of the drug. The pharmacological effects of prednisolone which are due to its glucocorticoid properties include: promotion of gluconeogenesis; increased deposition of glycogen in the liver; inhibition of the utilization of glucose; anti-insulin activity; increased catabolism of protein; increased lipolysis; stimulation of fat synthesis and storage; increased glomerular filtration rate and resulting increase in urinary excretion of urate (creatinine excretion)[5-9].Figure 1 the chemical structure of prednisolone; |
| Indication | It is indicated for the treatment of primary or secondary adrenocortical insufficiency[9], such as congenital adrenal hyperplasia, thyroiditis. It is also used to treat psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, bursitis, acute gouty arthritis and epicondylitis[10]; indicated for treatment of systemic lupus erythematosus, pemphigus and acute rhematic carditis; can be used in the treatment of leukemias, lymphomas, thrombocytopenia purpura and autoimmune hemolytic anemia; can be used to treat celiac disease, insulin resistance, ulcerative colitis and liver disorders. |
| Mechanism of action | Glucocorticoids such as Prednisolone can inhibit leukocyte infiltration at the site of inflammation, interfere with mediators of inflammatory response, and suppress humoral immune responses[10-13]. The antiinflammatory actions of glucocorticoids are thought to involve phospholipase A2 inhibitory proteins, lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes[10-13]. Prednisolone reduces inflammatory reaction by limiting the capillary dilatation and permeability of the vascular structures[14]. These compounds restrict the accumulation of polymorphonuclear leukocytes and macrophages and reduce the release of vasoactive kinins. Recent research suggests that corticosteroids may inhibit the release of arachidonic acid from phospholipids, thereby reducing the formation of prostaglandins. Prednisolone is a glucocorticoid receptor agonist [15]. On binding, the cortico-receptor-ligand complex translocates itself into the cell nucleus, where it binds to many glucocorticoid response elements (GRE) in the promoter region of the target genes. The DNA bound receptor then interacts with basic transcription factors, causing an increase or decrease in expression of specific target genes, including suppression of IL2 (interleukin 2) expression. |
| Pharmacokinetics | Prednisolone is cleared from the body primarily by hepatic metabolism, and greater than 90% of radioactivity administered orally or intravenously as[4-14] prednisolone is recovered in the urine[16,17]. Only approximately 7-15% of an oral dose of prednisone or prednisolone is excreted as unchanged prednisolone in the urine, the remainder being recovered as a variety of metabolites[18, 19]. The plasma half-life of prednisolone ranges from 2.5 to 3.5 hr(1). Similar half-life values for prednisolone are observed after oral prednisolone is administered[20, 21]. Mean plasma half-lives were 4.0 hr and 5.0 hr for the 12and 48-mg doses, respectively. Plasma clearance averaged 98.5-ml/min/1.73 m 2 and 120.1-ml/min/1.73 m 2 after these doses. Neither half-life nor clearance was statistically different between the two dose levels. Values for volume of distribution were calculated to be 21.6 and 27.7 liters /1.73 m 2 for Vl and 12.1 and 31.1 liters/1.73 m 2 for V2 with the 12and 48-mg doses, respectively. Only the differences in V2 were found to be statistically significant. It appears that prednisolone may exhibit dose-dependent pharmacokinetics, so that, with increasing dose, values for volume of distribution, plasma clearance, and half-life may increase. Although the exact reasons for these changes have not been established, they are believed to be related to changes in the plasma protein binding of prednisolone. It has been shown that prednisolone binds to plasma proteins (transcortin and albumin) in a nonlinear manner over the range of doses used, so that the percentage unbound increases with increasing dose[22]. This then leads to the observed changes in the pharmacokinetic parameters of prednisolone. |
| Adverse reactions | Various adverse reactions may be associated with the use of prednisolone[1, 10]. Common adverse reactions for corticosteroids include fluid retention, alteration in glucose tolerance, elevation in blood pressure, behavioral and mood changes, increased appetite and weight gain. Allergic Reactions: Anaphylaxis, angioedema. Cardiovascular: Bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, fat embolism, hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction, pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis Dermatologic: Acne, allergic dermatitis, cutaneous and subcutaneous atrophy, dry scalp, edema, facial erythema, hyper or hypo-pigmentation, impaired wound healing, increased sweating, petechiae and ecchymoses, rash, sterile abscess, striae, suppressed reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria Endocrine: Abnormal fat deposits, decreased carbohydrate tolerance, development of Cushingoid state, hirsutism, manifestations of latent diabetes mellitus and increased requirements for insulin or oral hypoglycemic agents in diabetics, menstrual irregularities, moon facies, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery or illness), suppression of growth in children Fluid and Electrolyte Disturbances: Fluid retention, potassium loss, hypertension, hypokalemic alkalosis, and sodium retention Gastrointestinal: Abdominal distention, elevation in serum liver enzymes levels (usually reversible upon discontinuation), hepatomegaly, hiccups, malaise, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, ulcerative esophagitis. General: Increased appetite and weight gain Metabolic: Negative nitrogen balance due to protein catabolism Musculoskeletal: Osteonecrosis of femoral and humeral heads, charcot-like arthropathy, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, steroid myopathy, tendon rupture, vertebral compression fractures. Neurological: Arachnoiditis, convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually following discontinuation of treatment, insomnia, meningitis, mood swings, neuritis, neuropathy, paraparesis/paraplegia, paresthesia, personality changes, sensory disturbances, vertigo. |
| Precaution | Alterations in Endocrine Function[10] Corticosteroids can produce reversible hypothalamic-pituitary adrenal (HPA) axis suppression with the potential for corticosteroid insufficiency after withdrawal of treatment. Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. If the patient is receiving corticosteroids already, dosage may have to be increased. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently. Mineralocorticoid supplementation is of particular importance in infancy. Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate adjustment in dosage. Corticosteroids may increase the risks related to infections with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic infections. The degree to which the dose, route and duration of corticosteroid administration correlates with the specific risks of infection is not well characterized, however, with increasing doses of corticosteroids, the rate of occurrence of infectious complications increases. Corticosteroids may mask some signs of infection and may reduce resistance to new infections. |
| Overdosage | The effects of accidental ingestion of large quantities of prednisone over a very short period of time have not been reported, but prolonged use of the drug can produce mental symptoms, moon face, abnormal fat deposits, fluid retention, excessive appetite, weight gain, hypertrichosis, acne, striae, ecchymosis, increased sweating, pigmentation, dry scaly skin, thinning scalp hair, increased blood pressure, tachycardia, thrombophlebitis, decreased resistance to infection, negative nitrogen balance with delayed bone and wound healing, headache, weakness, menstrual disorders, accentuated menopausal symptoms, neuropathy, fractures, osteoporosis, peptic ulcer, decreased glucose tolerance, hypokalemia, and adrenal insufficiency. Hepatomegaly and abdominal distention have been observed in children. Treatment of acute overdosage is by immediate gastric lavage or emesis followed by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy the dosage of prednisone may be reduced only temporarily, or alternate day treatment may be introduced. |
| Tips | The following tips should be followed when you plan to/are using prednisolone[1] You should not use prednisolone if you are allergic to it or have a fungal infection anywhere in your body. Prednisolone has the potential weaken your immune system, making it easier for you to get an infection. Steroids can also worsen an infection you already have, or reactivate an infection you recently had. You should keep consulting your doctors about any illness or infection you have had within the past several weeks. To make sure prednisolone is safe for you, let your doctor know if you have ever had active tuberculosis, a thyroid disorder, herpes infection of the eyes, stomach ulcers, ulcerative colitis, or diverticulitis, depression, mental illness, or psychosis, liver disease (especially cirrhosis), high blood pressure, osteoporosis, a muscle disorder such as myasthenia gravis; or multiple sclerosis. You should also inform your doctor if you have diabetes. Steroid medicines may further increase the glucose (sugar) levels in your blood or urine, worsening the situation of diabetes. You may also need to adjust the dose of your diabetes medications in that case. It is not known whether this medicine will harm an unborn baby. Tell your doctor if you are pregnant or plan to become pregnant. It is not known whether prednisolone passes into breast milk or if it could affect the nursing baby. Tell your doctor if you are breast-feeding. |
| Chemical Properties | Crystalline Solid |
| Originator | Sterane, Pfizer ,US ,1955 |
| Uses | Synthetic corticosteroid; metabolically interconvertible with prednisone |
| Uses | glucocorticoid |
| Uses | substance P antagonists (SPA). It mediates its effect by acting on neurokinin 1 receptor |
| Definition | ChEBI: A glucocorticoid that is prednisone in which the oxo group at position 11 has been reduced to the corresponding beta-hydroxy group. It is a drug metabolite of prednisone. Manufacturing Process The following procedure is described in US Patent 2,837,464: from a solution of 3 grams of yeast extract (Difco) in 3.0 liters of tap water containing 13.2 grams of potassium dihydrogen phosphate and 26.4 grams disodium hydrogen phosphate (pH of the solution, 6.9) 27 portions of 100 ml each are withdrawn, placed in 300 ml Erlenmeyer flasks and sterilized by autoclaving for 15 minutes at 15 pounds steam pressure (120°C). After autoclaving and cooling of the broth, one ml of suspension of Corynebacterium simplex (ATCC 6946) is placed in each flask. The flasks are then shaken on a shake table at 220 rpm and 28°C for 24 hours. Into each of 27 Erlenmeyer flasks are placed 150 mg of Kendall's Compound F (hydrocortisone). The flasks and contents are then sterilized for 15 minutes at 15 pounds steam pressure (120°C). To each flask are then added 5.0 ml of ethanol. The 24-hour bacterial culture is then transferred aseptically and the resulting suspensions are shaken on a shake table at 220 rpm and 28°C for 48 hours. The pH at the end of the shake period is 7.0. The contents of all the flasks are combined and extracted with a total of 9.0 liters of chloroform in 3 equal portions. The combined extracts are then concentrated to a residue which weighs 3.75 grams. The MP of the residue is 227°-232°C. From 2.75 grams of this crude material on sludging with 50 ml of acetone and cooling, there is recovered on filtration 1.35 grams of ?1,4pregnadiene-11β,17α,21-triol-3,20-dione,MP 237°-239°C (dec.). Additional product can be recovered from the mother liquor. Recrystallization from acetone raised the MP to 239°-241°C (dec.). |
| Brand name | Cortalone(Halsey); Delta-Cortef (Pharmacia & Upjohn); Fernisolone(Ferndale); Meti-Derm (Schering); Prelone (Muro); Prelone(Teva); Sterane (Pfizer). |
| Therapeutic Function | Glucocorticoid |
| General Description | Prednisolone,Δ1-hydrocortisone,11β,17,21-trihydroxypregna-1,4-diene-3,20-dione, hasless salt-retention activity than hydrocortisone, but some patients have more frequently experiencedcomplications such as gastric irritation and peptic ulcers.Because of low MC activity, it cannot be used alone for adrenalinsufficiency. Prednisolone is available in varioussalts and esters to maximize its therapeutic utility: Prednisolone acetate, USP (21-acetate) Prednisolone sodium phosphate, USP (21-sodiumphosphate) Prednisolone sodium succinate, USP (21-sodiumsuccinate) Prednisolone tebutate, USP (21-tebutate). |
| Hazard | Causes sodium retention; may have side effects similar to cortisone. |
| Mechanism of action | Prednisolone is hydrocortisone to which has been added a ?1 double bond. This places two double bonds in ring A, which flattens it and increases glucocorticoid action at the expense of mineralocorticoid activity. Prednisolone has fourfold the glucocorticoid activity of hydrocortisone while having approximately half its mineralocorticoid activity. In addition, prednisolone has an increased duration of action compared to hydrocortisone, because the extra double bond in ring A retards its metabolic reduction. |
| Clinical Use | Prednisolone can be used to treat severe asthmatic attacks that do not respond to conventional treatment, and it is available as the free alcohol for oral administration. The C-21 sodium phosphate (Hydeltrasol) ester is available for parenteral use. |
| Side effects | A prodrug of prednisolone is prednisone. It is the 11-keto analogue of prednisolone and must be converted in vivo to the active 11β-hydroxy compound, which is necessary to hydrogen bond to Asn-564 in the glucocorticoid receptor. Prednisone should not be used in patients with hepatic dysfunction, because their ability to reduce the 11-keto group with 11β-hydroxysteroid dehydrogenase to the active metabolite may be impaired. |
| Safety Profile | A poison by intravenous and subcutaneous routes. Moderately toxic by ingestion and intraperitoneal routes. Human teratogenic effects by an unspecified route: developmental abnormalities of the central nervous system; effects on embryo or fetus: fetal death, extra embryonic structures. Human reproductive effects by an unspecified route: stdlbirth. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Veterinary Drugs and Treatments | Glucocorticoids have been used in an attempt to treat practically every malady that afflicts man or animal, but there are three broad uses and dosage ranges for use of these agents. |
| Prednisolone Preparation Products And Raw materials | |
|---|---|
| Raw materials | Hydrocortisone
|
| Preparation Products | Prednicarbate-->11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-methanesulphonate |
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.