
| Name | Deflazacort |
|---|---|
| Synonyms | 16-d)oxazole-3,20-dione,11-beta,21-dihydroxy-2’-5’-beta-h-pregna-4-dieno(17; azacort; calcort; deflan; (5'β)-11β-Hydroxy-21-acetyloxy-2'-methyl-1,2,4,5-tetradehydropregnano[17,16-d]oxazole-3,20-dione; (5'β)-21-Acetyloxy-11β-hydroxy-2'-methylpregnano[17,16-d]oxazole-1,4-diene-3,20-dione; 11b,21-Dihydroxy-2'-; Denazacort deflan l-5458 azacort calcort Dezacor Azaeort Caltort MDL 458 Oxazaoon lantadin Dezacort L 5458-d3 dl-458-it Flantadin Lalltadin Deflan-d3 Dezacor-d3 Azacort-d3 Calcort-d3 MDL 458-d3 Denazacort Deflzacort Lantadin-d3 Dezacort-d3 DL 458IT-d3 DEFLAZACORT Flantadin-d3 Oxazacort-d3 DECYLACRYLATE Azacortinol-d3 Deflazacort-d3 Dezacor:Flantadin Deflazacort(Calcort) 11b,21-Dihydroxy-2'- Deflazacort (100 mg) Deflazacort solution,100ppm Calcort, Deflan, Dezacor, Flantadin, Lantadin 16-d)oxazole-3,20-dione,11-beta,21-dihydroxy-2’-5’-beta-h-pregna-4-dieno(17 pregna-1,4-diene-11β,21-diol-3,20-dione[17α,16α-d]-2′-methyloxazoline 21-acetate (5'β)-21-Acetyloxy-11β-hydroxy-2'-methylpregnano[17,16-d]oxazole-1,4-diene-3,20-dione 11b,21-dihydroxy-2'-methyl-5'bh-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione 21-acetate 11β,21-dihydroxy-2′-methyl-5′βH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione 2′-acetate 5'bH-Pregna-1,4-dieno[17,16-d]oxazole-3,20-dione, 11b,21-dihydroxy-2'-methyl-, 21-acetate (11,16)-21-(acetyloxy)-11-hydroxy-2'-methyl-5'H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione 11 BETA 21 DIHYDROXY-2-METHYL-5 BETAH-PREGNA-1,4-DIENO (17,16-D) OXAZOLE-3,20-DIONE21-ACETATE (1lβ,16β)-21-(Acetyloxy)-11-hydroxy-2’-methyl-5’H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione (5'β)-11β-Hydroxy-21-acetyloxy-2'-methyl-1,2,4,5-tetradehydropregnano[17,16-d]oxazole-3,20-dione (11β,16β)-21-(Acetyloxy)-11-hydroxy-2'-Methyl-5'H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione-d3 5'H-Pregna-1,4-dieno[17,16-d]oxazole-3,20-dione, 21-(acetyloxy)-11-hydroxy-2'-methyl-, (11b,16b)- 5'H-Pregna-1,4-dieno[17,16-d]oxazole-3,20-dione, 21-(acetyloxy)-11-hydroxy-2'-methyl-, (11β,16β)- |
| CAS NO | 14484-47-0 |
| EINECS | 238-483-7 |
| Molecular Weight | 441.52 |
| Molecular Formula | C25H31NO6 |
| Product Categories | CALCORT; Steroid and Hormone; Intermediates & Fine Chemicals; Pharmaceuticals;Steroids |
| Mol File | 14484-47-0.mol |
Deflazacort Chemical Properties | |
|---|---|
| Melting point | 255-256.5°C |
| Boiling point | 595.4±50.0 °C(Predicted) |
| density | 1.41 |
| storage temp | -20°C Freezer |
| pka | 14.30±0.70(Predicted) |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| color | white to tan |
| Merck | 14,2862 |
| Safety Information | |
|---|---|
| Safety Statements | 24/25 |
| WGK Germany | 3 |
| RTECS | TU4157050 |
| HS Code | 29349990 |
| Toxicity | LD50 orally in mice: 5200 mg/kg (Schiatti) |
| Deflazacort Usage And Synthesis | |
|---|---|
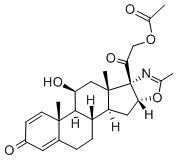
| Description | Deflazacort (trade name Emflaza among others) is a glucocorticoid used as an anti-inflammatory and immunosuppressant. It belongs to a group of medicines called corticosteroids. It is sometimes referred to simply as an oral steroid. Deflazacort is an inactive prodrug which is metabolized rapidly to the active drug 21-desacetyldeflazacort. It is used to treat a wide variety of conditions. Some examples include autoimmune diseases (for example, systemic lupus erythematosus (SLE), autoimmune hepatitis, sarcoidosis), joint and muscle diseases (for example, rheumatoid arthritis), and allergies and asthma. Cardiff can be specially designed for the third generation of corticosteroids, anti-inflammatory, anti-allergy, and it increased gluconeogenesis and so on. It acts as prednisolone 10~20 times of the dragon, 40 times as hydrocortisone. This product has no mutagenic effect. This product, labeled with C14 study, shows that the product of the gastrointestinal tract rapidly absorbed, reaching the peak blood concentration after 1~2h, forming the active substance by hydrolysis, and the latter further metabolic processes is similar with other corticosteroids, as well as the active metabolite T1/2 is 2 h and the rats are mainly distributed in the kidney and hematopoietic system and other organizations. Figure 1 is a deflazacort formula. Chemical Properties Acetone-hexane, m.p. 255-256.5 ℃. [Α] D + 62.3 ° (C = 0.5, chloroform). UV absorption maximum (methanol): 241~242nm (E1cm1% 352.5). Acute toxicity LD50 in mice (mg/kg): 5200 oral; 1610 subcutaneous injection. Rats Acute toxicity LD50 (mg/kg): 109 subcutaneously. Dog Acute toxicity LD50 (mg/kg):> 4000 Oral; 50 subcutaneous injection. |
| Chemical Properties | Acetone-hexane, m.p. 255-256.5 ℃. [Α] D + 62.3 ° (C = 0.5, chloroform). UV absorption maximum (methanol): 241~242nm (E1cm1% 352.5). Acute toxicity LD50 in mice (mg/kg): 5200 oral; 1610 subcutaneous injection. Rats Acute toxicity LD50 (mg/kg): 109 subcutaneously. Dog Acute toxicity LD50 (mg/kg):> 4000 Oral; 50 subcutaneous injection. |
| Application | The third generation of corticosteroids, anti-inflammatory, anti-allergy, increased gluconeogenesis and so on. For the use of the primary and secondary adrenocortical insufficiency, rheumatism, collagen diseases, skin diseases, allergic diseases, eye diseases, and fulminant disseminated tuberculosis, hematopoietic system disorders, ulcerative colitis, idiopathic nephrotic syndrome, and other hematopoietic malignancies. |
| Adverse effects | Deflazacort carries the risks common to all corticosteroids, including immune suppression, decreased bone density, and endocrine insufficiency. In clinical trials, the most common side effects (>10% above placebo) were Cushing's-like appearance, weight gain, and increased appetite. Long-term medication can affect the normal growth of children, induced peptic ulcer perforation, which can cause euphoria, depression, insomnia and other psychiatric symptoms. Sudden withdrawal after long-term use can cause secondary adrenal insufficiency withdrawal reactions, which should gradually decrease until disabled. |
| Precautions | Like other glucocorticoids, it is general contraindicated in systemic infections. • Used with caution in diverticulitis, recent gastrointestinal surgery, renal failure, hypertension, diabetes, osteoporosis, myasthenia gravis embolism. • Pregnancy and lactation women should used with caution. |
| Drug interactions | Cardiff may have unique row potassium, and it should pay more attention when combined with diuretics. |
| Preparation | Compound 10g (1) was dissolved in 350ml methanol, and then added 8.3g of semicarbazide hydrochloride and 5.75ml of pyridine in 50ml of water, after the reaction. We can obtain compound 11g (Ⅱ). Compound 9g (Ⅱ) was dissolved 230ml 95% ethanol, the solution was added 3.6g of potassium carbonate and 2.34g of sodium borohydride in 36ml of water, which was refluxed for 30min and then added 2.34g of sodium borohydride. After the reaction, we can obtain compound 8.5g (Ⅲ). Compound 7.7g (Ⅲ) was dissolved in 154ml 10% hydrochloric acid in methanol, refluxed for 1h, to give Compound 6g (Ⅳ). Compound 12.5g (Ⅳ) was dissolved in 500ml of anhydrous toluene and 90ml of cyclohexanone. It was added 6.24g triisopropyl aluminum, 9g obtained by reacting the compound (V). 6.5g compound (V) was dissolved in 123ml dry dioxane, and 25% hydrogen bromide in acetic acid solution is heated, and then added 5.5g of bromine in dioxane; the reaction solution was poured into the water, collected by filtration after the crystals were carefully dried, redissolved in dimethylformamide. Itwas added 7.5g of lithium bromide and lithium carbonate (1: 2) mixture. After heating the reaction, we can obtain the compound 5.1g (ⅵ). 2g compound (Ⅵ) was dissolved in tetrahydrofuran-methanol (1: 1), added to 3.9g calcium oxide and 0.1g of azobisisobutyronitrile. It was added 2g of iodine in tetrahydrofuran-methanol mixture; After the reaction was filtered off precipitate , which was dissolved in acetone,it was then added triethylamine 20ml, 20ml and 12ml acetone, acetic acid, atter the reaction, we can obtain 1.6g deflazacort. |
| References | https://en.wikipedia.org/wiki/Deflazacort http://patient.info/medicine/deflazacort-tablets-calcort |
| Description | Deflazacort is an antiinflammatory treatment of rheumatoid arthritis. Its antirheumatic potency i s similar to that of prednisone, while its hyperglycernic and calcium depleting effects are reportedly less. |
| Chemical Properties | Off-White to Pale Yellow Solid |
| Originator | Dow Lepetit (Italy) |
| Uses | A systemic corticosteroid. A derivative of prednisolone.Used for rheumatoid arthritis and lupus |
| Uses | antiinflammatory |
| Brand name | LANTADIN; DEFLAN |
<p
| Deflazacort Preparation Products And Raw materials | |
|---|---|
| Raw materials |
|
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.