
| Name | Fluticasone propionate |
|---|---|
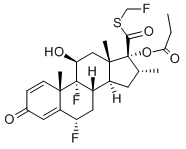
| Synonyms | s-fluoromethyl-6alpha,9alpha-difluoro-11beta-hydroxy-16alpha-methyl-3-oxo-17alpha-propionyloxyandrosta-1,4-diene-17beta-carbothioate; -17-(1-oxopropoxy)-,s-(fluoromethyl)ester,(6-alpha,11-beta,16-alpha,17-alph;androsta-1,4-diene-17-carbothioicacid,6,9-difluoro-11-hydroxy-16-methyl-3-oxo; cci18781; fluticasone17-propionate; flutide; (6A,11B,16A,17A)-6,9-DIFLUORO-11-HYDROXY-16-METHYL-3-OXO-17-(1-OXOPROPOXY)ANDROSTA-1,4-DIENE-17-CARBOTHIOIC ACID FLUOROMETHYL ESTER; (6 ALPHA,11 BETA,16 ALPHA,17 ALPHA)-6,9-DIFLUORO-11-HYDROXY-16-METHYL-3-OXO-17-(1-OXOPROPOXY)ANDROSTA-1,4-DIENE-17-CARBOTHIOIC ACID FLUOROMETHYL ESTER (6A,11B,16A,17A)-6,9-DIFLUORO-11-HYDROXY-16-METHYL-3-OXO-17-(1-OXOPROPOXY)ANDROSTA-1,4-DIENE-17-CARBOTHIOIC ACID FLUOROMETHYL ESTER (6 ALPHA,11 BETA,16 ALPHA,17 ALPHA)-6,9-DIFLUORO-11-HYDROXY-16-METHYL-3-OXO-17-(1-OXOPROPOXY)ANDROSTA-1,4-DIENE-17-CARBOTHIOIC ACID FLUOROMETHYL ESTER (6ALPHA, 11BETA, 16ALPHA, 17ALPHA)-6,9-DIFLUORO-11-HYDROXY-16-METHYL-3-OXO-17-(1-OXOPROPOXY)ANDROSTA-1,4-DIENE-17-CARBOTHIOIC ACID S-(FLUOROMETHYL) ESTER CCI-187881 CUTIVATE FLIXONASE FLIXOTIDE FLONASE FLOVENT FLUNASE FLUTICASONE-D5 PROPIONATE FLUTICASONE PROPIONATE s-fluoromethyl-6alpha,9alpha-difluoro-11beta-hydroxy-16alpha-methyl-3-oxo-17alpha-propionyloxyandrosta-1,4-diene-17beta-carbothioate -17-(1-oxopropoxy)-,s-(fluoromethyl)ester,(6-alpha,11-beta,16-alpha,17-alph androsta-1,4-diene-17-carbothioicacid,6,9-difluoro-11-hydroxy-16-methyl-3-oxo cci18781 fluticasone17-propionate flutide Fluticasone Propionate-C25H31F3O5S Fluticasonepropiponate |
| CAS NO | 80474-14-2 |
| EINECS | 80474-14-2 |
| Molecular Weight | 500.57 |
| Molecular Formula | C25H31F3O5S |
| Product Categories | HIVID; Hormone Drugs; Steroid and Hormone; Intermediates & Fine Chemicals; Pharmaceuticals; Fluticasone; Biochemistry; Hydroxyketosteroids; Steroids |
| Mol File | 80474-14-2.mol |
Fluticasone propionate Chemical Properties | |
|---|---|
| Melting point | 275 °C |
| Boiling point | 568.3±50.0 °C(Predicted) |
| alpha | D +30° (c = 0.35) |
| refractive index | 31 ° (C=1, Dioxane) |
| storage temp | Store at RT |
| pka | 12.53±0.70(Predicted) |
| solubility | DMSO: ≥10 mg/mL |
| form | solid |
| color | white |
| Merck | 14,4211 |
| InChIKey | WMWTYOKRWGGJOA-CENSZEJFSA-N |
| CAS DataBase Reference | 80474-14-2(CAS DataBase Reference) |
| EPA Substance Registry System | Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-,S-(fluoromethyl) ester, (6.alpha.,11.beta.,16.alpha.,17.alpha.)- (80474-14-2) |
| Safety Information | |
|---|---|
| Safety Statements | 22-24/25 |
| WGK Germany | 2 |
| RTECS | BV7980000 |
| Toxicity | LD50 oral in rat: > 2gm/kg |
| HS Code | 29372900 |
| Fluticasone propionate Usage And Synthesis | |
|---|---|
| Description | Fluticasone propionate is a new glucocorticosteroid useful in the treatment of seasonal rhinitis and asthma. Compared to beclomethasone dipropionate, fluticasone propionate is reported to have a comparable side effect profile, but twice the activity in the treatment of asthma. |
| Chemical Properties | Crystalline Solid |
| Originator | Glaxo (United Kingdom) |
| Uses | A derivative of Flumethasone. An antiallergic; anti-asthmatic; anti-inflammatory. |
| Uses | antiviral |
| Uses | Atorvastatin Intermediate |
| Uses | An antiallergic and anti inflammatory |
| Uses | A high-affinity, selective glucocorticoid receptor agonist |
| Definition | ChEBI: A trifluorinated corticosteroid that consits of 6alpha,9-difluoro-11beta,17alpha-dihydroxy-17beta-{[(fluoromethyl)sulfanyl]carbonyl}-16-methyl-3-oxoandrosta-1,4-diene bearing a propionyl s bstituent at position 17; has anti-inflammatory, anti-asthmatic and anti-allergic activity. |
| Manufacturing Process | A solution of 6α,9α-difluoro-11β,17α-dihydroxy-16α-methyl-3-oxoandrosta1,4-diene-7β-carboxylic acid (2.113 g) and triethylamine (2.5 ml) in dichloromethane (60 ml) was treated at 0°C with propionyl chloride (1.85 ml). After 1 h the mixture was diluted with more solvent (50 ml) and washed successively with 3% sodium hydrogen carbonate, water, 2 N hydrochloric acid, water, saturated brine, then dried and evaporated. The solid was dissolved in acetone (50 ml) and diethylamine (2.5 ml) was added. After 1 h at 22°C the solvent was removed in vacuo and the residual gum was dissolved in water (30 ml). Acidification to pH 1 with 2 N hydrochloric acid precipitated a solid, which was collected, washed with water, and dried to give the 6α,9αdifluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene17β-carboxylic acid (2.230 g), melting point 220-225°C, [α]D =+4° (c 0.70) A solution of the 6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17αpropionyloxyandrosta-1,4-diene-17β-carboxylic acid (500 mg) and sodium iodide (1.874 g) in acetone (15 ml) was stirred and heated under reflux for 6.5 h. Ethyl acetate (75 ml) was then added and the solution was washed successively with water, 10% sodium thiosulfate solution, 5% sodium hydrogen carbonate solution and water, dried and evaporated to give an offwhite foam (525 mg). PLC in chloroform-acetone (6:1) to give an off-white foam (478 mg) which was crystallised from acetone without being heated above room temperature to give colourless crystals of the S-iodomethyl6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4diene-17β-carbothioate (241 mg); m.p. 196-197°C, [α]D = -32° (c 1.01). A solution of S-iodomethyl-6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo17α-propionyloxyandrosta-1,4-diene-17β-carbothioate (310 mg) in acetonitrile (10 ml) was stirred with silver fluoride (947 mg) for 3 days at room temperature in the dark. Ethyl acetate (100 ml) was added and the mixture was filtered through kieselguhr. The filtrate was washed successively with 2 N hydrochloric acid, water, saturated brine, then dried. The solvent was removed and the residue was subjected to column chromatography in chloroform then chloroform-acetone (19:1). The product was eluted with ethyl acetate and crystallised on concentration of the solution to give S-fluoromethyl 6α,9αdifluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene17β-carbothioate (0.075 g); melting point 272-273°C (dec.), [α]D= +30° (c 0.35). |
| Brand name | Cutivate (Altana); Flonase (GlaxoSmithKline); Flovent (GlaxoSmithKline);Flixonase. |
| Therapeutic Function | Glucocorticoid |
| General Description | General Description Fluticasone propionate,S-(fluoromethyl) 6α,9-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-(1-oxopropoxy)androsta-1,4-diene-17-carbothio-ate (Cutivate), is threefold to fivefold more potentthan dexamethasone in receptor binding assays. |
| General Description | The main metabolite of fluticasonepropionate (Flovent, Flonase) found in circulationin humans is the 17β-carboxylate derivative. As expected,a charged carboxylate in place of the normal acetol functionalityat C17 greatly reduces affinity for the GR (2,000-fold less than the parent), and this metabolite is essentiallyinactive. The metabolite is formed via the CYP3A4 system,so care should be taken if fluticasone propionate iscoadministered with a CYP3A4 inhibitor such as ketoconazoleor ritonavir. Clinically induced Cushing syndromehas been observed when inhaled fluticasone propionatewas administered concurrently with ritonavir.Fluticasone is also available in an inhaled formulation incombination with the long-acting β2-agonist salmeterol(Advair Diskus). |
| Biological Activity | High affinity, selective glucocorticoid receptor agonist (K d = 0.5 nM). Potently stimulates glucocorticoid receptor-mediated transactivation of gene expression and enhances human eosinophil apoptosis (EC 50 = 3.7 nM) in vitro . Inhibits mast cell accumulation in nasal mucosa following topical administration. Lipophilic, clinically-used anti-inflammatory agent with low oral bioavailability. |
| Mechanism of action | Fluticasone propionate has a unique C-20 thioflouromethyl group, and that, in combination with the 17α-propionate ester, gives it 36-fold the glucocorticoid receptor affinity as compared to beclomethasone dipropionate and twofold the affinity as compared to budesonide. The 9α-flouro group increases both glucocorticoid and mineralocorticoid activities, and the 6α-flouro group enhances only the glucocorticoid action. Studies have determined that the effectiveness of inhaled fluticasone propionate results from a local rather than a systemic effect. |
| Clinical Use | As a consequence of its high lipophilicity, fluticasone propionate is very insoluble and, therefore, is poorly absorbed from the respiratory (10–13%) and GI tract following nasal inhalation of the drug (112,113,114). The majority of the intranasal dose for fluticasone propionate is deposited in the nasal mucosa and swallowed into the GI tract until eliminated in the feces (~80–90% of the intranasal dose is recovered metabolized and unchanged in the feces). After administration of an IV suspension, fluticasone propionate displayed a systemic bioavailability of less than 2% and underwent extensive hydrolysis and CYP3A4 first-pass metabolism in the liver, with an elimination half-life of approximately 3 hours. Its primary hydrolysis product is 17β-carboxylate metabolite, which has 1/2,000 the affinity for the GR, can be recovered from the urine along with other unidentified hydroxy metabolites and their conjugates. Following oral administration of 1 to 40 mg, fluticasone propionate is poorly absorbed from the GI tract because of hydrolysis and its insolubility, with an oral bioavailability of less than 1%. Less than 5% of the oral dose is excreted unchanged in the urine. |
| Veterinary Drugs and Treatments | While there are topical forms of fluticasone, most veterinary interests are in the inhaled versions of the drug. The aerosol for pulmonary inhalation appears to be effective in treating feline asthma, recurrent airway obstruction (RAO, heaves) or inflammatory airway disease (IAD) in horses, and dogs with chronic tracheobronchial disease. While the majority of small animal use has been with fluticasone, there are several other aerosol corticosteroids for inhalation (beclomethasone dipropionate, flunisolide, and triamcinolone acetonide) that theoretically could be used for the same purpose. The nasal inhalation corticosteroid products may be useful for allergyrelated chronic rhinosinusitis in cats and dogs |
| Metabolism | Interestingly, less than 1% of the swallowed dose is bioavailable, in contrast to the fact that the majority of the inhaled dose is systemically available and highly protein bound. Fluticasone propionate is not a prodrug and is extensively metabolized by the liver. The only detectable metabolite is the 17β-carboxylic acid derived from CYP3A4 oxidation. This metabolite has 2,000-fold less affinity for the glucocorticoid receptor than the parent drug. Elimination is through both the feces and the urine, with the relative amounts determined by the route of administration. Fluticasone propionate is available in aerosol and powder inhalation formulations. |
| Fluticasone propionate Preparation Products And Raw materials | |
|---|---|
| Raw materials | Dimethyl sulfide-->3-METHOXYPROPIONIC ACID-->Bromofluoromethane-->Sodium iodide-->Silver fluoride
|
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.