
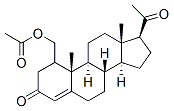
| Name | Medroxyprogesterone 17-acetate |
|---|---|
| Synonyms | 17a-hydroxy-6a-methyl-4-pregnene-3,20-dione acetate 17alpha-acetoxy-6-alpha-methylpregn-4-ene-3,20-dione 17ALPHA-ACETOXY-6ALPHA-METHYLPROGESTERONE 17ALPHA-HYDROXY-6ALPHA-METHYL-4-PREGNENE-3,20-DIONE 17-ACETATE 17-ALPHA-HYDROXY-6-ALPHA-METHYL-4-PREGNENE-3,20-DIONE 17-ALPHA-ACETATE 4-PREGNEN-6ALPHA-METHYL-17ALPHA-OL-3,20-DIONE 17-ACETATE 4-PREGNEN-6-ALPHA-METHYL-17-OL-3,20-DIONE ACETATE 6-ALPHA-METHYL-17-ACETOPROGESTERONE 6ALPHA-METHYL-17ALPHA-ACETOXYPROGESTERONE 6ALPHA-METHYL-17ALPHA-HYDROXY-PROGESTERONE ACETATE DEPO-PROVERA hydroxymethylprogesterone acetate MEDROXYPROGESTERONE 17-ACETATE MEDROXYPROGESTERONE ACETATE MPA PROVERA (6-alpha)-17-(acetyloxy)-6-methylpreg-4-ene-3,20-dione 17-(acetyloxy)-6-methyl-(6-alpha)-pregn-4-ene-20-dione 17-(acetyloxy)-6-methyl-20-dion(6alpha)-pregn-4-ene- 17-acetoxy-6-alpha-methylprogesterone |
| CAS NO | 71-58-9 |
| EINECS(EC#) | 200-757-9 |
| Molecular Formula | C24H34O4 |
| MDL Number | MFCD00010483 |
| Molecular Weight | 386.52 |
| MOL File | 71-58-9.mol |
| Appearance | white crystalline powder |
| mp | 206-207 °C(lit.) |
| refractive index | 48 ° (C=1, Dioxane) |
| Stability | Stable, but weakly air and light sensitive. Incompatible with strong oxidizing agents |
| Water Solubility | <0.1 g/100 mL at 23 ºC |
| Merck | 13,5817 |
| EPA Substance Registry System | 71-58-9(EPA Substance) |
| CAS DataBase Reference | 71-58-9(CAS DataBase Reference) |
| Medroxyprogesterone Acetate Chemical Properties | |
|---|---|
| Melting point | 206-207 °C(lit.) |
| alpha | D +61° (in chloroform) |
| Boiling point | 432.7°C (rough estimate) |
| density | 1.0346 (rough estimate) |
| refractive index | 48 ° (C=1, Dioxane) |
| storage temp | Refrigerator |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in acetone, sparingly soluble in ethanol (96 per cent) |
| form | neat |
| Water Solubility | <0.1 g/100 mL at 23 ºC |
| Merck | 13,5817 |
| BRN | 2066112 |
| Stability | Stable, but weakly air and light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 71-58-9(CAS DataBase Reference) |
| IARC | 2B (Vol. 21, Sup 7) 1987 |
| EPA Substance Registry System | Medroxyprogesterone acetate (71-58-9) |
| Safety Information | |
|---|---|
| Hazard Codes | Xn |
| Risk Statements | 40-48 |
| Safety Statements | 22-36/37/39-45 |
| WGK Germany | 3 |
| RTECS | TU5010000 |
| HS Code | 29372390 |
| Medroxyprogesterone Acetate Usage And Synthesis | |
|---|---|
| Chemical Properties | White or almost white, crystalline powder |
| Originator | Provera,Upjohn,US,1959 |
| Uses | Progestogen; an injectable contraceptive |
| Uses | antiprotozoal |
| Uses | A synthetic progesterone receptor agonist |
| Manufacturing Process | Preparation of 17α-Hydroxyprogesterone 3,20-Bis-(Ethylene Ketal): A solution was prepared containing 50.0 g of 17α-hydroxyprogesterone in 1,000 ml of benzene, 100 ml of ethylene glycol and 2.5 g of p-toluenesulfonic acid monohydrate. This mixture was refluxed for a period of 17 hours using a calcium carbide water-trap to remove the water formed in the reaction. After this period of reflux 6.5 ml of pyridine was added to the solution, and the mixture cooled to room temperature. The lower glycol layer was separated and washed with benzene. The benzene layer and the benzene washings were combined and the combined solution was divided into two equal portions, one of which was used for the isolation of 17α-hydroxyprogesterone 3,20-bis-(ethylene ketal) as follows. The benzene solution was washed with 5% sodium carbonate solution, water and saturated sodium chloride solution. After being dried over anhydrous magnesium sulfate the solution was concentrated to dryness at reduced pressure, The residue was recrystallized by taking up in hot methylene chloride, adding acetone and boiling to remove the methylene chloride until a final volume of about 200 ml was reached. |
| Brand name | Amen (Amarin); Curretab (Solvay Pharmaceuticals); Cycrin (ESI); Provera (Pharmacia & Upjohn);Clinovie;Cliovir;Dep0-clinover;Dep0-map;Depcorlutin;Depo-prodasone;Depo-progevera;Depo-promone;Deporone;Dugen;Farlurin;Farlutale;Gesinal;Gestapuran;Gestapuron;G-farlutal;Hysron;Intex;Luteocrin orale;Luteodione;Luteos;Lutoporal;Metigestene;Nadigest;Nogest;Onco-provera;Perlutest;Petogen;Piermap;Povera;Promone-e;Pronone;Proverone;Provest;Sindomens;Sodelut "g";Supprestal;Verafen;Veramix plus v. |
| Therapeutic Function | Progestin |
| World Health Organization (WHO) | A depot preparation containing 150 mg medroxyprogesterone acetate was introduced over 20 years ago for use as a long-acting injectable contraceptive. Subsequently, positive results of carcinogenicity studies carried out in beagle bitches led to refusal of registration in the United States. These findings were later considered irrelevant to contraceptive use in women and the drug was approved by the Food and Drug Administration. Menstrual irregularities are the most common adverse effect associated with depot medroxyprogesterone acetate. Risk-benefit judgements differ significantly from country to country, having regard to differing national circumstances. The preparation is, however, widely available and is included in the WHO Model List of Essential Drugs. |
| General Description | PA, 17-acetyloxy-6α-methylpregn-4-ene-3,20-dione (Provera), adds a6α-methyl group to the basic 17α-hydroxyprogesteronestructure to greatly decrease the rate of reduction of the 4-ene-3-one system. The 17α-acetate group also decreases reductionof the 20-one, similar to the 17α-caproate. MPA isvery active orally and has such a long durationof action intramuscularly that it cannot be routinely usedintramuscularly for treating many menstrual disorders. |
| General Description | Odorless white to off-white microcrystalli |
| Air & Water Reactions | Medroxyprogesterone 17-acetate is sensitive to prolonged exposure to air and light. Insoluble in water. |
| Hazard | Possible carcinogen |
| Fire Hazard | Flash point data for Medroxyprogesterone 17-acetate are not available; however, Medroxyprogesterone 17-acetate is probably combustible |
| Safety Profile | Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Human systemic effects by intravenous route: increased intraocular pressure. Human teratogenic effects by an unspecified route: developmental abnormalities of the urogenital systemHuman reproductive effects by multiple routes: spermatogenesis, menstrual cycle changes or dlsorders, postpartum effects, female fertility effects, abortion, newborn behavioral effects. Human mutation data reported. Experimental reproductive effects. A drug for the treatment of secondary amenorrhoea and dysfunctional uterine bleeding. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Veterinary Drugs and Treatments | In cats, MPA has been used when either castration is ineffective or undesirable to treat sexually dimorphic behavior problems such as roaming, inter-male aggressive behaviors, spraying, mounting, etc. MPA has also been used as a tranquilizing agent to treat syndromes such as feline psychogenic dermatitis and alopecia, but treatment with “true” tranquilizing agents may be preferable. In humans, parenteral MPA has been used as a long-acting contraceptive in females, to decrease sexually deviant behavior in males, and as an antineoplastic agent for some carcinomas (see Pharmacology section above). Oral MPA is used in human females to treat secondary amenorrhea and to treat abnormal uterine bleeding secondary to hormone imbalances. |
| Metabolism | Among the first of these substituted 17α-acetoxyprogesterone analogues to be utilized therapeutically was medroxyprogesterone acetate, a 6α-methyl progesterone analogue. This analogue is 25-fold more active than ethisterone. Following oral administration, medroxyprogesterone acetate is completely and rapidly deacetylated by first-pass metabolism to medroxyprogesterone. Medroxyprogesterone is extensively metabolized via pathways similar to those for progesterone, except for 6α-hydroxylation. Most medroxyprogesterone acetate metabolites are excreted in the urine, primarily as glucuronide conjugates. Plasma protein binding for medroxyprogesterone is approximately 86%, primarily to serum albumin, with no binding to SHBG. |
Medroxyprogesterone Acetate Preparation Products And Raw materials | |
|---|---|
| Raw materials | Sulfuric acid-->Ethylene glycol-->Peroxyacetic acid-->METHYLMAGNESIUM BROMIDE-->Acetic anhydride |
FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.