
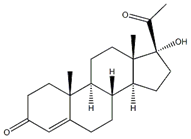
| Name | 17a-Hydroxyprogesterone (HPG) Hydroxyprogesterone |
|---|---|
| Synonyms | 17-Hydroxypregn-4-en-3,20-dione; 17-hydroxy-pregn-4-ene-20-dione; 17-hydroxypregn-4-ene-3,20-dione; delta(4)-pregnene-17alpha-ol-3,20-dione; gestagenogador; prodix; prodox; (8R,9S,10R,13S,14S,17R)-17-ACETYL-17-HYDROXY-10,13-DIMETHYL-1,2,6,7,8,9,10,11,12,13,14,15,16,17-TETRADECAHYDRO-CYCLOPENTA[A]PHENANTHREN-3-ONE 17A-HYDROXY-4-PREGNENE-3,20-DIONE 17a-hydroxypregn-4-ene-3,20-dione 17A-HYDROXYPROGESTERONE 17ALPHA-HYDROXY-4-PREGNENE-3,20-DIONE 17-ALPHA-HYDROXYPREGN-4-ENE-3,20-DIONE 17ALPHA-HYDROXYPROGESTERONE 17-HYDROXYPROGESTERONE 4-PREGNEN-17ALPHA-OL-3,20-DIONE 4-pregnen-17a-ol-3,20-dione 4-PREGNEN-17-OL-3,20-DIONE (8R,9S,10R,13S,14S,17R)-17-ACETYL-17-HYDROXY-10,13-DIMETHYL-1,2,6,7,8,9,10,11,12,13,14,15,16,17-TETRADECAHYDRO-CYCLOPENTA[A]PHENANTHREN-3-ONE HYDROXYPROGESTERONE HYDROXYPROGESTERONE, 17A- 17-Hydroxypregn-4-en-3,20-dione 17-hydroxy-pregn-4-ene-20-dione 17-hydroxypregn-4-ene-3,20-dione delta(4)-pregnene-17alpha-ol-3,20-dione gestagenogador prodix prodox |
| CAS NO | 68-96-2 |
| MF | C21H30O3 |
| MW | 330.46 |
| EINECS | 200-699-4 |
| Product Categories | Inhibitors; MIRADON; Steroids; Biochemistry; Hydroxyketosteroids; Intermediates & Fine Chemicals; Pharmaceuticals |
| Mol File | 68-96-2.mol |
| Hydroxyprogesterone Chemical Properties | |
|---|---|
| Melting point | 276°C |
| Boiling point | 407.89°C (rough estimate) |
| density | 1.0998 (rough estimate) |
| refractive index | 90 ° (C=1, CHCl3) |
| Fp | 9℃ |
| storage temp | -20?C Freezer |
| pka | 13.03±0.60(Predicted) |
| form | neat |
| Water Solubility | 5.056mg/L(20 ºC) |
| Merck | 14,4839 |
| BRN | 3218109 |
| CAS DataBase Reference | 68-96-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Pregn-4-ene-3,20-dione, 17-hydroxy-(68-96-2) |
| Safety Information | |
|---|---|
| Hazard Codes | T,F |
| Risk Statements | 61-39/23/24/25-23/24/25-11 |
| Safety Statements | 53-22-36/37/39-45-36/37-16 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | TU5060000 |
| HS Code | 38220090 |
| Hazardous Substances Data | 68-96-2(Hazardous Substances Data) |
| Hydroxyprogesterone Usage And Synthesis | |
|---|---|
| Chemical Properties | White Solid |
| Originator | Prodox,Upjohn |
| Uses | Progesteron. It was isolated from adrenal glands |
| Uses | anticoagulant |
| Uses | 17α-Hydroxy Progesterone is a metabolite of Progesterone. It was isolated from adrenal glands |
| Definition | ChEBI: A 17alpha-hydroxy steroid that is the 17alpha-hydroxy derivative of progesterone. |
| Indications | Hydroxyprogesterone has been used prophylactically for the 12th to 37th week of pregnancy, particularly in women who are in the high-risk category for premature delivery (e.g., those with a history of premature delivery or spontaneous abortion). A concern relating to teratogenic potential has limited its use. Hydroxyprogesterone as a tocolytic agent requires further evaluation before its routine prophylactic administration can be recommended. |
| Manufacturing Process | A suspension of 90.0 g of δ5-pregnen-3β,17α-diol-20-one in 2300 ml of 85% formic acid was shaken for 2 h at a temperature of 70C. During this time the compound partially dissolved and at the same time a new crystalline substance appeared in the solution. After cooling, the precipitate was filtered, thus giving 80.0 g of the 3-formate of δ5-pregnen-3β,17α-diol-20-one having a melting point of 204°-207°C. 5.0 g of the 3-formate of δ5-pregnen-3β,17α-diol-20-one suspended in 120 ml of acetic anhydride was treated with 1.5 g of p-toluenesulfonic acid and the mixture was stirred for 9 h at room temperature. It was poured into water and after 2 h standing, the precipitate was filtered and washed to neutral, thus yielding the 3-formate 17-acetate of δ5-pregnen-3β,17α-diol-20-one in a yield of over 90%. 1.0 g of 3-formate 17-acetate of δ5-pregnen-3β,17α-diol-20-one was dissolved in 30 ml of xylene and 10 ml of cyclohexanone and 4 ml of the solution were distilled in order to remove traces of moisture. 1.0 g of aluminum isopropylate was added to the hot solution and the mixture was refluxed for 45 min. After cooling to 90°C, water was added and the organic solvents were removed by steam distiliation. Salt was added to the aqueous suspension and the residue was filtered, dried and extracted with hot acetone. The acetone solution was evaporated to dryness and the residue was crystallized from chloroformmethanol, thus giving 610.0 mg of the 17-acetate of δ4-pregnen-17α-ol-3,20- dione (17-acetoxy-progesterone) with a melting point of 239°-240°C. Saponification of this compound with 1% methanolic potassium hydroxide yielded 80% of δ4-pregnen-17α-ol-3,20-dione |
| Therapeutic Function | Progestin. |
| Hydroxyprogesterone Preparation Products And Raw materials | |
|---|---|
Raw materials | Diosgenin-->Formic acid-->Aluminium isopropoxide-->Potassium hydroxide-->Acetic anhydride-->p-Toluenesulfonic acid |
| Preparation Products | 17-hydroxy-6-methylenepregn-4-ene-3,20-dione 17-acetate-->Megestrol acetate-->3,20-Dioxopregn-4-en-17-beta-yl acetate-->6-Dibromomethylene-17-hydroxypregn-4-ene-3,20-dione 17-acetate |
FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.