
| Name | Stanolone Dihydrotestosterone (DHT) |
|---|---|
| Synonyms | Androstanolone Protona |
| CAS NO | 521-18-6 |
| MF | C19H30O2 |
| MW | 290.44 |
| EINECS | 208-307-3 |
| Product Categories | API; Intermediates & Fine Chemicals; Pharmaceuticals; Steroid and Hormone; Steroids |
| Mol File | 521-18-6.mol |
| Stanolone Chemical Properties | |
|---|---|
| Melting point | 178-183 °C |
| alpha | 27 º |
| Boiling point | 372.52°C (rough estimate) |
| density | 1.0320 (rough estimate) |
| refractive index | 1.4709 (estimate) |
| Fp | 9℃ |
| storage temp | Controlled Substance, -20°C Freezer |
| form | neat |
| pka | 15.08±0.60(Predicted) |
| Water Solubility | 344.3g/L(temperature not stated) |
| Merck | 13,8872 |
| BRN | 2056371 |
| InChIKey | NVKAWKQGWWIWPM-ABEVXSGRSA-N |
| CAS DataBase Reference | 521-18-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Stanolone(521-18-6) |
| EPA Substance Registry System | Androstan-3-one, 17-hydroxy-, (5.alpha.,17.beta.)- (521-18-6) |
| Safety Information | |
|---|---|
| Hazard Codes | Xn,T,F |
| Risk Statements | 61-40-39/23/24/25-23/24/25-11 |
| Safety Statements | 53-36/37/39-45-36/37-16 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | BV8052000 |
| Stanolone Usage And Synthesis | |
|---|---|
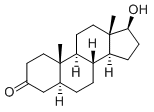
| Description | Stanolone is well known as dihydrotestosterone (DHT), which is an endogenous androgen sex steroid and hormone. |
| Chemical Properties | White Crystalline Solid |
| Originator | Neodrol,Pfizer,US,1953 |
| Uses | Anabolic steroid. Controlled substance |
| Definition | ChEBI: A 17beta-hydroxy steroid that is testosterone in which the 4-5 double bond has been reduced to a single bond with alpha- configuration at position 5 |
| Manufacturing Process | A solution of 1.0 g of 3,17-androstandione in 50 ml of methanol and containing 1 g of selenium dioxide, was allowed to remain in an ice-chest overnight. The formed 3,3-dimethoxyandrostan-17-one was not separated. 1 g of solid potassium hydroxide and 2.5 g of sodium borohydride in 2.5 ml of water were added and the mixture allowed to react at room temperature for 24 hours. The solution was then poured into a large excess of water, extracted with methylene chloride, the organic layer dried and evaporated to a residue. The residue was dissolved in ether, and a small amount of selenium removed by filtration. The ether was boiled off and the organic material dissolved in 100 ml of boiling acetone. 25 ml of diluted hydrochloric acid were added, the solution boiled for 5 minutes and then allowed to cool. Upon crystallization, 0.85 g of androstan-17β-ol-3-one was obtained, melting point 175°C to 178°C. |
| Therapeutic Function | Androgen |
| References | https://en.wikipedia.org/wiki/Dihydrotestosterone#Medical_use Swerdloff, R. S., and C. Wang. "Dihydrotestosterone: a rationale for its use as a non-aromatizable androgen replacement therapeutic agent."Baillieres Clin Endocrinol Metab 12.3(1998):501. |
FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.