
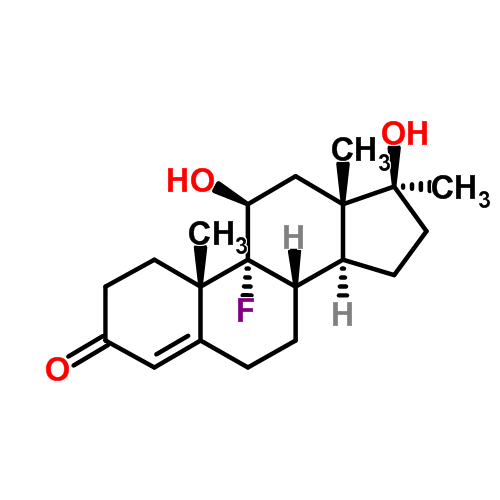
| Name | Fluoxymesterone |
|---|---|
| CAS NO | 76-43-7 |
| EINECS | 200-961-8 |
| Molecular Formula | C20H29FO3 |
| Molecular Weight | 336.4409 |
| Other Name | 9-alpha-fluoro-11-beta,17-beta-dihydroxy-17-alpha-methylandrost-4-en-3-one (11beta,17beta)-9-fluoro-11,17-dihydroxy-17-methylandrost-4-en-3-one fluoxymesterone standard solution fluoxymesterone—dea schedule iii 11BETA,17BETA-DIHYDROXY-9ALPHA-FLUORO-17ALPHA-METHYL-4-ANDROSTEN-3-ONE 4-ANDROSTEN-9-ALPHA-FLUORO-17-ALPHA-METHYL-11-BETA, 17-BETA-DIOL-3-ONE 9a-fluoro-11b,17b-dihydroxy-17a-methyl-4-androsten-3-one 9ALPHA-FLUORO-11BETA-HYDROXY-17ALPHA-METHYLTESTOSTERONE FLUOXYMESTERONE 11-beta,17-beta-Dihydroxy-9-alpha-fluoro-17-alpha-methyl-4-androster-3-one 17-alpha-methyl-9-alpha-fluoro-11-beta-hydroxytesterone 17-alpha-Methyl-9-alpha-fluoro-11-beta-hydroxytestosterone 9-alpha-fluoro-11-beta,17-beta-dihydroxy-17-alpha-methyl-4-androstene-3-one 9alpha-Fluoro-11beta,17beta-dihydroxy-17alpha-methyl-4-androstene-3-one 9-alpha-fluoro-11-beta-hydroxy-17-methyltestosterone 9alpha-Fluoro-11beta-hydroxy-17-methyltestosterone 9-alpha-Fluoro-17-alpha-methyl-11-beta,17-dihydroxy-4-androsten-3-one 9-fluoro-11,17-dihydroxy-17-methyl-,(11-beta,17-beta)-androst-4-en-3-on 9-fluoro-11,17-dihydroxy-17-methyl-,(11beta,17beta)-androst-4-en-3-on 9-Fluoro-11,17-dihydroxy-17-methylandrost-4-en-3-one 9-fluoro-11-beta,17-beta-dihydroxy-17-methyl-androst-4-en-3-on 9-fluoro-11beta,17beta-dihydroxy-17-methyl-androst-4-en-3-on 9-Fluoro-11-beta,17-beta-dihydroxy-17-methylandrost-4-en-3-one Androfluorene |
| Density | 1.22g/cm3 |
| Melting Point(℃) | 240℃ |
| Boiling Point | 474.2°C at 760 mmHg |
| Vapour Pressure | 5.5E-11mmHg at 25°C |
| Flash Point | 240.6°C |
| Refractive Index | 1.562 |
| Fluoxymesterone Chemical Properties | |
|---|---|
| Melting point | 240 °C |
| alpha | 104 º (c=1,EtOH) |
| Boiling point | 474.2±45.0 °C(Predicted) |
| density | 1.0455 (estimate) |
| storage temp | 20°C |
| solubility | H2O: ≤0.5 mg/mL |
| pka | 13.40±0.70(Predicted) |
| form | solid (photosensitive) |
| color | white |
| Water Solubility | NEGLIGIBLE |
| Merck | 13,4212 |
| CAS DataBase Reference | 76-43-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Androsten-3-one, 9alpha-fluoro-11beta,17beta-dihydroxy-17alpha-methyl-,(76-43-7) |
| EPA Substance Registry System | Fluoxymesterone (76-43-7) |
| Safety Information | |
|---|---|
| Hazard Codes | Xn,T,F |
| Risk Statements | 63-38-19-11-61-60 |
| Safety Statements | 22-36-24/25-45-53 |
| WGK Germany | 3 |
| RTECS | BV8390000 |
| HS Code | 29372900 |
| Hazardous Substances Data | 76-43-7(Hazardous Substances Data) |
| Toxicity | LD50 intraperitoneal in mouse: 2350mg/kg |
| Fluoxymesterone Usage And Synthesis | |
|---|---|
| Chemical Properties | white to light yellow crystal powde |
| Originator | Halotestin, Upjohn, US,1957 |
| Uses | Fluoxymesterone is an anabolic steroid with androgenic activity. Fluoxymesterone is used in the treatment of male hypogonadism. Fluoxymesterone showed antitumor effects on pregnancy-dependent mammary tumors TPDMT-4. |
| Uses | stimulates erythropoesis and cell respiration treatment of male hypogonadismdelayed puberty in males, managing metastatic breast cancer in menopausal women |
| Manufacturing Process | The following description is taken from US Patent 2,793,218. (a) Preparation of 9/(11)-Dehydro-17-Methyltestosterone: A warm solution of 1 gram of 11α-hydroxy-17-methyltestosterone (US Patent 2,660,586) in 2 ml of dry pyridine was mixed with 1 gram of para-toluenesulfonyl chloride. The mixture was maintained at room temperature for 18 hours and then poured into 25 ml of water. The mixture was stirred until the precipitated oil solidified. The solid was filtered, washed with water and dried to give 1.41 grams of 11α-(p-toluenesulfonyloxy)-17α-methyl-17β-hydroxy-4-androsten-3-one which melted at 144° to 148°C with decomposition and, after crystallization from a mixture of methylene chloride and hexane hydrocarbons, melted at 141° to 144°C with decomposition. A mixture of 1 gram of the thus-produced 11α-(p-toluenesulfonyloxy)-17αmethyl-17β-hydroxy-4-androsten-3-one, 0.2 gram of sodium formate, 0.57 ml of water and 14 ml of absolute ethanol was heated at its refluxing temperature for 19 hours. The solution was cooled and then poured onto 50 grams of a mixture of ice and water with stirring. The resulting precipitate was filtered and dried to give 0.59 gram of 9(11)-dehydro-17methyltestosterone which melted at 156° to 160°C and, after crystallization from a mixture of methylene chloride and hexane hydrocarbons, melted at 167° to 170°C. (b) Preparation of 9α-Bromo-11β-Hydroxy-17-Methyltestosterone: To a solution of 1 gram of 9(11)-dehydro-17-methyltestosteronein 50 ml of acetone was added dropwise, with stirring, at 15°C, 1 gram of Nbromoacetamide dissolved in 25 ml of water. A solution of 20 ml of 0.8 N perchloric acid was then slowly added at the same temperature. After 20 minutes, there was added a sufficient amount of a saturated aqueous solution of sodium sulfite to discharge the yellow color of the solution. The resulting mixture was then diluted with 100 ml of water thereby precipitating 1 gram of 9α-bromo-11β-hydroxy-17-methyltestosterone as needles melting at 153° to 155°C. (c) Preparation of 9,11β-Epoxy-17-Methyltestosterone: A suspension of 1 gram of 9α-bromo-11β-hydroxy-17-methyltestosterone in 30 ml of methanol was titrated with 1 M equivalent of 0.1 N aqueous sodium hydroxide. The resulting mixture was diluted with 50 ml of water and then chilled to about 0°C thereby precipitating 0.64 gram of 9,11β-epoxy-17-methyltestosterone melting at 170° to 176°C which, after crystallization from dilute methanol, melted at 65° to 172°C (with sublimation). (d) Preparation of 9α-Fluoro-11β-Hydroxy-17-Methyltestosterone: To a solution of 0.5 gram of 9,11β-epoxy-17-methyltestosterone in 10 ml of methylene chloride was added 2 ml of 48% aqueous hydrofluoric acid. The mixture was stirred at room temperature for 5 hours and then cautiously poured with stirring into a mixture of 6 grams of sodium bicarbonate in a mixture of ice and water. The precipitated steroid was extracted with methylene chloride, the extract washed with water and then dried. The solvent was distilled from the dried solution and the residue crystallized from methylene chloride to give 148 mg of 9α-fluoro-11β-hydroxy-17methyltestosterone melting at 265°C with decomposition. |
| Brand name | Android (Valeant); Halotestin (Pharmacia & Upjohn); Ora-Testryl (Bristol-Myers Squibb) |
| Therapeutic Function | Androgen |
| General Description | Fluoxymesterone, 9α-fluoro-11β,17β-dihydroxy-17-methylandrost-4-en-3-one, is ahighly potent, orally active androgen, about 5 to 10 timesmore potent than testosterone. It can be used for all theindications discussed previously, but its great androgenicactivity has made it useful primarily for treatment of theandrogen-deficient male. |
| Pharmacokinetics | By substituting a 9α-fluoro group onto an analog of 17α-methyltestosterone, fluoxymesterone has 20 times the anabolic and 10 times the androgenic activity of 17α-methyltestosterone. It has a mean half-life of 9 hours, and less than 5% of the drug is excreted unchanged. An adverse effect of fluoxymesterone is sodium and water retention that could lead to edema. |
| Safety Profile | Poison by ingestion. Moderatelytoxic by intraperitoneal route. Human systemic effects byingestion: dermatitis, changes in respiratory system andtransaminase activity. Human reproductive effects byingestion: spermatogenesis. An experimental teratogen. |
FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost


Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Designed by HuishangMedia
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved
Design by Huishang Media
Under CC: ultimatearm, Freepik, Nhor Phai, DinosoftLabs, Vitaly Gorbachev, Kiranshastry, Pixel perfect
If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately.